Page 97 - Williams Hematology ( PDFDrive )
P. 97
72 Part II: The Organization of the Lymphohematopoietic Tissues Chapter 5: Structure of the Marrow and the Hematopoietic Microenvironment 73
Figure 5–9. Transmission electron micrograph of mouse
femoral marrow. A. The lumen (L) of a marrow sinus is indi-
L L cated. The arrow points to the thin endothelial cytoplas-
mic lining of the sinus. The nucleus of a megakaryocyte
(N) is indicated, with the cytoplasm of the megakaryocyte
invaginating the endothelial cell cytoplasm in three places
below the lumen. B. The arrow indicates the thin endo-
thelial cell cytoplasmic lining of the sinus. The endothe-
lium is attenuated to a double membrane in two places.
A small process of megakaryocyte cytoplasm has formed
a pore in the endothelial cell and has entered the sinus
lumen (L). Cytoplasm flows through such pores and deliv-
ers proplatelets to the sinus lumen. (Used with permission of
MA Lichtman, University of Rochester.)
N
A A B
stress of moderate blood loss also increases the cell cycling of the HSCs, the degradation of CXCL12 by neutrophil-associated enzymes such
but those cycling HSCs cannot be detected in the blood, indicating as neutrophil elastase, cathepsin G, and MMP-9 or the HSC enzyme
552
that the migration of HSCs in response to stress is very likely related CD26/dipeptidylpeptidase, but mice genetically null for the proteases
to the inflammatory/injury component of the stress. This relation- or treated with other protease inhibitors still show the G-CSF–induced
ship between inflammation/injury and HSC migration has been used decrease of CXCL12 mRNA and protein. 480,553,556,557 Multiple mechanisms
experimentally to understand the mechanisms of HSC migration into for CXCL12 modulation have been proposed, including the adrenergic
the blood and clinically to mobilize the HSCs into the blood for collec- nervous system suppressing MSC production of CXCL12 and direct
tion for use in stem cell transplantation. Not surprisingly, these studies G-CSF suppression of osteoblast lineage cells in the marrow. 140,141,557,558
demonstrate that much of the regulation of HSC migration involves the The successful development of small antagonists of CXCR4, such as
reversal or inhibition of the mechanisms by which the HSCs home to plerixafor (formerly AMD3100), has provided a rapid means to mobi-
the marrow and develop quiescence. lize HSCs and is used clinically for those patients that fail to mobilize
Many hematopoietic growth factors can mobilize HSCs from the with G-CSF. Similarly, blocking α -integrin binding or genetic dele-
421
4
marrow to the blood, but the best understood and most used clinically tion of the α -integrin component leads to HSC mobilization within
4
is G-CSF. 480,506,553 Similar to other growth factors, the G-CSF mobiliza- 1 or 2 days under both homeostatic or G-CSF–induced conditions.
421
tion of HSCs requires several days for maximal effect. A major deter- This mobilization appears to be mainly mediated through disruption
minant in both the homing to and migration from the marrow is the of VLA-4 activity and is further enhanced by blocking other adhesion
interaction of CXCR4 on HSCs with its ligand CXCL12 in the marrow. mediators such as the β -integrins or E-selectin, neither of which has an
2
G-CSF induces stem cell mobilization by decreasing CXCL12 signal- effect when used alone. 421,559 Some of β -integrin’s synergistic effects may
2
ing. CXCR4 knockout mice do not mobilize HSCs with G-CSF, but be indirect through the action on other cells. HSC mobilization with
554
560
they mobilize HSCs in response to VLA-4 (α β integrin) antagonists. antibodies against the α component of integrin is replicated by potent
561
555
4
4 1
Inhibitor studies originally identified the mobilization mechanism as and selective small molecule antagonists. The results of interfering
562
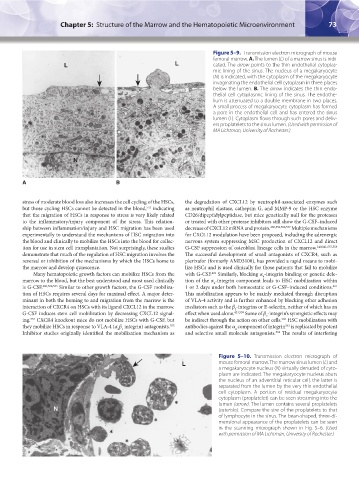
Figure 5–10. Transmission electron micrograph of
L mouse femoral marrow. The marrow sinus lumen (L) and
a megakaryocyte nucleus (N) virtually denuded of cyto-
plasm are indicated. The megakaryocyte nucleus abuts
the nucleus of an adventitial reticular cell; the latter is
separated from the lumen by the very thin endothelial
cell cytoplasm. A portion of residual megakaryocyte
∗ cytoplasm (proplatelet) can be seen streaming into the
lumen (arrow). The lumen contains several proplatelets
N (asterisks). Compare the size of the proplatelets to that
∗ of lymphocyte in the sinus. The bean-shaped, three-di-
mensional appearance of the proplatelets can be seen
∗ in the scanning micrograph shown in Fig. 5–6. (Used
with permission of MA Lichtman, University of Rochester.)
∗
Kaushansky_chapter 05_p0051-0084.indd 73 9/19/15 12:11 AM