Page 96 - Williams Hematology ( PDFDrive )
P. 96
72 Part II: The Organization of the Lymphohematopoietic Tissues Chapter 5: Structure of the Marrow and the Hematopoietic Microenvironment 73
A number of releasing factors are implicated in the initiation of Releasing factors for reticulocytes have been difficult to identify.
530
marrow granulocyte egress, including G-CSF, 528,529 GM-CSF, the C3e Adventitial reticular cell cytoplasm is a barrier to the reticulocytes on
544
531
component of complement, zymosan-activated plasma-containing the abluminal surface of the endothelium. Phlebotomy, phenylhy-
532
complement fragments, glucocorticoid hormones, androgenic ste- drazine-induced hemolytic anemia, and EPO result in marked reduc-
533
535
534
roids, and endotoxin. Neutrophils residing in the marrow venous tion of the adventitial cell cover of the sinus, a process that is thought to
536
sinusoids are rapidly released into the circulation by IL-8. In a rat facilitate cell egress through the endothelium. Immature reticulocytes
545
model in which releasing factors can be given through the femoral have much less deformability than more mature ones, suggesting that
546
artery and neutrophils collected from the femoral vein, chemokines active migration by nascent reticulocytes through the endothelial cells
CXCL2 (MIP-2) and CXCL1 (KC) that are produced at sites of inflam- is relatively unlikely, and release is via a passive mechanism. Thus, retic-
mation induce rapid, selective neutrophil migration from the marrow ulocytes appear to require a pressure gradient to cross the venous endo-
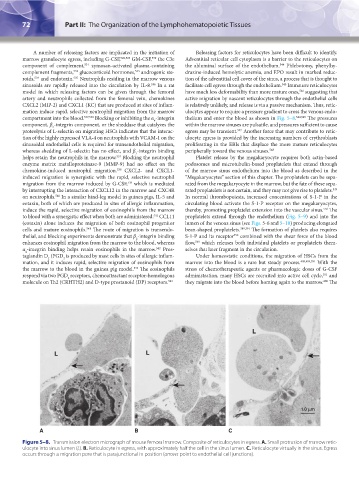
compartment into the blood. 537,538 Blocking or inhibiting the α -integrin thelium and enter the blood as shown in Fig. 5–8. 544,545 The pressures
4
component, β -integrin component, or the sheddase that catalyzes the within the marrow sinuses are pulsatile, and pressures sufficient to cause
2
proteolysis of L-selectin on migrating HSCs indicates that the interac- egress may be transient. Another force that may contribute to retic-
547
tion of the highly expressed VLA-4 on neutrophils with VCAM-1 on the ulocyte egress is provided by the increasing numbers of erythroblasts
sinusoidal endothelial cells is required for transendothelial migration, proliferating in the EBIs that displace the more mature reticulocytes
whereas shedding of L-selectin has no effect, and β -integrin binding peripherally toward the venous sinuses. 548
2
537
helps retain the neutrophils in the marrow. Blocking the neutrophil Platelet release by the megakaryocyte requires both actin-based
enzyme matrix metalloproteinase-9 (MMP-9) had no effect on the podosomes and microtubulin-based proplatelets that extend through
538
chemokine-induced neutrophil migration. CXCL2- and CXCL1- of the marrow sinus endothelium into the blood as described in the
induced migration is synergistic with the rapid, selective neutrophil “Megakaryocytes” section of this chapter. The proplatelets can be sepa-
539
migration from the marrow induced by G-CSF, which is mediated rated from the megakaryocyte in the marrow, but the fate of these sepa-
549
by interrupting the interaction of CXCL12 in the marrow and CXC4R rated proplatelets is not certain, and they may not give rise to platelets.
on neutrophils. In a similar hind-leg model in guinea pigs, IL-5 and In normal thrombopoiesis, increased concentrations of S-1-P in the
540
eotaxin, both of which are produced in sites of allergic inflammation, circulating blood activate the S-1-P receptor on the megakaryocytes,
induce the rapid, selective migration of eosinophils from the marrow thereby, promoting proplatelet extension into the vascular sinus. The
550
541
to blood with a synergistic effect when both are administered. CCL11 proplatelets extend through the endothelium (Fig. 5–9) and into the
(eotaxin) alone induces the migration of both eosinophil progenitor lumen of the venous sinus (see Figs. 5-6 and 5–10) producing elongated
541
cells and mature eosinophils. The route of migration is transendo- bean-shaped proplatelets. 389,391 The formation of platelets also requires
550
thelial, and blocking experiments demonstrate that β -integrin binding S-1-P and its receptor combined with the shear force of the blood
2
549
enhances eosinophil migration from the marrow to the blood, whereas flow, which releases both individual platelets or proplatelets them-
α -integrin binding helps retain eosinophils in the marrow. Pros- selves that later fragment in the circulation.
542
4
taglandin D (PGD is produced by mast cells in sites of allergic inflam- Under homeostatic conditions, the migration of HSCs from the
2
2
mation, and it induces rapid, selective migration of eosinophils from marrow into the blood is a rare but steady process. 408,409,551 With the
the marrow to the blood in the guinea pig model. The eosinophils stress of chemotherapeutic agents or pharmacologic doses of G-CSF
543
respond via two PGD receptors, chemoattractant receptor-homologous administration, many HSCs are recruited into active cell cycle, and
551
2
molecule on Th2 (CRHTH2) and D-type prostanoid (DP) receptors. 543 they migrate into the blood before homing again to the marrow. The
408
L
L L
1.0 µm
A B C
Figure 5–8. Transmission electron micrograph of mouse femoral marrow. Composite of reticulocytes in egress. A. Small protrusion of marrow retic-
ulocyte into sinus lumen (L). B. Reticulocyte in egress, with approximately half the cell in the sinus lumen. C. Reticulocyte virtually in the sinus. Egress
occurs through a migration pore that is parajunctional in position (arrows point to endothelial cell junctions).
Kaushansky_chapter 05_p0051-0084.indd 72 9/19/15 12:11 AM