Page 1090 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1090
1054 ParT EighT Immunology of Neoplasia
15
Traditionally, CNS leukemia has been defined as the presence analysis. In most centers, karyotype by classic cytogenetics (see
of at least five leukocytes per microliter of cerebrospinal fluid Fig. 78.2) is performed. However, because of the lack of meta-
(CSF) and the detection of leukemic blast cells, by the presence phases, the yield of karyotypic analysis of ALL in multicenter
of cranial nerve palsy, or by retinal involvement, as detected by protocols is often <70%. Furthermore, normal karyotypes are
ophthalmoscopy. Although overt CNS leukemia is relatively rare, sometimes derived from the normal cells in bone marrow rather
submicroscopic CNS involvement is present at diagnosis in at than the leukemia blasts. This drawback can be overcome by
least half the patients in the absence of any neurological symp- interphase fluorescent in-situ hybridization (FISH), a technique
toms. Thus CNS-directed therapy is routinely included in ALL that does not require metaphases. All clinically relevant structural
therapy. and numerical chromosomal aberrations can be detected with
The differential diagnosis of ALL includes neoplastic and the use of commercially available FISH probes (Fig. 78.3). Fusion
nonneoplastic diseases. Because children with ALL present with translocations, such as BCR–ABL, TEL–AML1, and MLL–AF4,
a variety of nonspecific symptoms, several pediatric nonmalignant can all be detected by using reverse transcription–polymerase
conditions may be confused with leukemia. Since treatment with chain reaction (RT-PCR).
steroids may mask the presence of ALL, serious consideration The elucidation of the human genome and the invention of
of the diagnosis of ALL must be given before starting treatment the genomic technologies are in the process of revolutionizing
with steroids to any pediatric nonmalignant disorder. Bone the diagnostics of leukemias. New methodologies of next-
marrow examination is recommended in case of uncertainty. generation genome sequencing (NGS) are likely to transform
Idiopathic thrombocytopenic purpura (ITP) is a common both our understanding of leukemia biology and the diagnostic
cause of bruising and petechiae in children. ITP is characterized approach. Routine use of NGS mutation panels and copy number
by the absence of any other hematological abnormalities. Bone genomic arrays are likely to replace routine cytogenetic analysis
marrow should be examined if anemia or hepatosplenomegaly in the near future.
are present.
Infectious mononucleosis may present with fever, malaise,
adenopathy, splenomegaly, rash, and lymphocytosis. The atypical
lymphocytes may morphologically resemble the leukemic
lymphoblasts. Rarely, flow cytometry may be necessary to dis-
tinguish between the activated atypical lymphocytes and the
immature leukemic lymphoblasts.
Leukemoid reactions, observed in sepsis, acute hemolysis, and
other disorders, are usually easy to distinguish from ALL by
morphological examination of peripheral blood smear. Since
occasionally ALL presents with pancytopenia, aplastic anemia
is also on the differential diagnosis list. A B
CLiNiCaL PEarLS
Acute Lymphoblastic Leukemia (ALL) and der(21)
Rheumatoid Disorders
• ALL can mimic juvenile idiopathic arthritis (JIA; Chapter 53) and other
musculoskeletal disorders.
• Because leukemic blasts may be absent from the peripheral blood,
bone marrow examination should be considered in any child with JIA, der(12)
especially prior to commencing steroid therapy.
As many as 10% of children with ALL are first evaluated at
pediatric rheumatology clinics. Fever, arthralgias, arthritis, or a
limp accompanied by anemia, mild splenomegaly, and lymph-
adenopathy frequently can be confused with juvenile idiopathic
arthritis (Chapter 53) or osteomyelitis. These patients may be C
treated with antibiotics and antiinflammatory agents for several
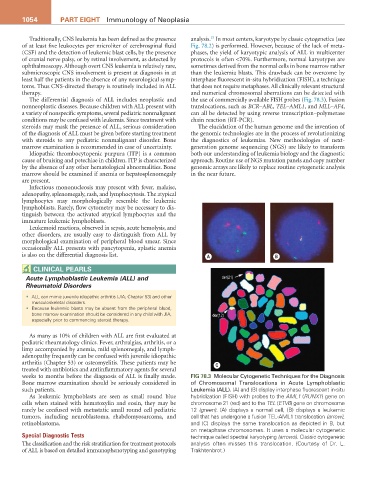
weeks to months before the diagnosis of ALL is finally made. Fig 78.3 Molecular Cytogenetic Techniques for the Diagnosis
Bone marrow examination should be seriously considered in of Chromosomal Translocations in Acute Lymphoblastic
such patients. Leukemia (ALL). (A) and (B) display interphase fluorescent in-situ
As leukemic lymphoblasts are seen as small round blue hybridization (FISH) with probes to the AML1 (RUNX1) gene on
cells when stained with hematoxylin and eosin, they may be chromosome 21 (red) and to the TEL (ETV6) gene on chromosome
rarely be confused with metastatic small round cell pediatric 12 (green). (A) displays a normal cell, (B) displays a leukemic
tumors, including neuroblastoma, rhabdomyosarcoma, and cell that has undergone a fusion TEL-AML1 translocation (arrow),
retinoblastoma. and (C) displays the same translocation as depicted in B, but
on metaphase chromosomes. It uses a molecular cytogenetic
Special Diagnostic Tests technique called spectral karyotyping (arrows). Classic cytogenetic
The classification and the risk stratification for treatment protocols analysis often misses this translocation. (Courtesy of Dr. L.
of ALL is based on detailed immunophenotyping and genotyping Trakhtenbrot.)