Page 1089 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1089
ChaPTEr 78 Lymphoid Leukemias 1053
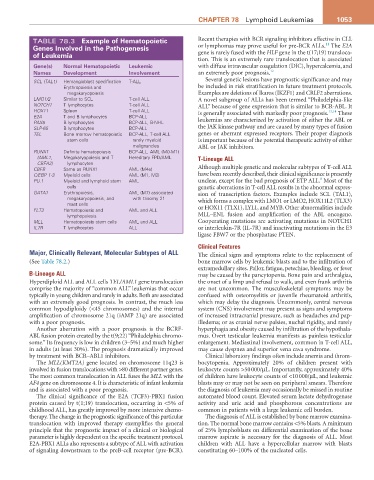
TABLE 78.3 Example of hematopoietic Recent therapies with BCR signaling inhibitors effective in CLL
11
genes involved in the Pathogenesis or lymphomas may prove useful for pre-BCR ALLs. The E2A
of Leukemia gene is rarely fused with the HLF gene in the t(17;19) transloca-
tion. This is an extremely rare translocation that is associated
gene(s) Normal hematopoietic Leukemic with diffuse intravascular coagulation (DIC), hypercalcemia, and
Names Development involvement an extremely poor prognosis. 12
SCL (TAL1) Hemangioblast specification T-ALL Several genetic lesions have prognostic significance and may
Erythropoiesis and be included in risk stratification in future treatment protocols.
megakaryopoiesis Examples are deletions of Ikaros (IKZF1) and CRLF2 aberrations.
LMO1/2 Similar to SCL T-cell ALL A novel subgroup of ALLs has been termed “Philadelphia-like
NOTCH1 T lymphocytes T-cell ALL ALL” because of gene expression that is similar to BCR-ABL. It
HOX11 Spleen T-cell ALL is generally associated with markedly poor prognosis. 13,14 These
E2A T and B lymphocytes BCP-ALL
PAX5 B lymphocytes BCP-ALL, B-NHL leukemias are characterized by activation of either the ABL or
SLP-65 B lymphocytes BCP-ALL the JAK kinase pathway and are caused by many types of fusion
TEL Bone marrow hematopoietic BCP-ALL, T-cell ALL genes or aberrant expressed receptors. Their proper diagnosis
stem cells rarely myeloid is important because of the potential therapeutic activity of either
malignancies ABL or JAK inhibitors.
RUNX1 Definite hematopoiesis BCP-ALL, AML (M0-M1)
(AML1, Megakaryopoiesis and T Hereditary FPD/AML T-Lineage ALL
CBFA2) lymphocytes
CBFB Same as RUNX1 AML (M4e) Although multiple genetic and molecular subtypes of T-cell ALL
C/EBP 1-3 Myeloid cells AML (M1, M2) have been recently described, their clinical significance is presently
8
PU.1 Myeloid and lymphoid stem AML unclear, except for the bad prognosis of ETP ALL. Most of the
cells genetic aberrations in T-cell ALL results in the abnormal expres-
GATA1 Erythropoiesis, AML (M7) associated sion of transcription factors. Examples include SCL (TAL1),
megakaryopoiesis, and with trisomy 21 which forms a complex with LMO1 or LMO2, HOX11L2 (TLX3)
mast cells
FLT3 Hematopoiesis and AML and ALL or HOX11 (TLX1), LYL1, and MYB. Other abnormalities include
lymphopoiesis MLL–ENL fusion and amplification of the ABL oncogene.
MLL Hematopoiesis stem cells AML and ALL Cooperating mutations are activating mutations in NOTCH1
IL7R T lymphocytes ALL or interleukin-7R (IL-7R) and inactivating mutations in the E3
ligase FBW7 or the phosphatase PTEN.
Clinical Features
Major, Clinically Relevant, Molecular Subtypes of ALL The clinical signs and symptoms relate to the replacement of
(See Table 78.2.) bone marrow cells by leukemic blasts and to the infiltration of
extramedullary sites. Pallor, fatigue, petechiae, bleeding, or fever
B-Lineage ALL may be caused by the pancytopenia. Bone pain and arthralgias,
Hyperdiploid ALL and ALL cells TEL/AML1 gene translocation the onset of a limp and refusal to walk, and even frank arthritis
comprise the majority of “common ALL” leukemias that occur are not uncommon. The musculoskeletal symptoms may be
typically in young children and rarely in adults. Both are associated confused with osteomyelitis or juvenile rheumatoid arthritis,
with an extremely good prognosis. In contrast, the much less which may delay the diagnosis. Uncommonly, central nervous
common hypodiploidy (<45 chromosomes) and the internal system (CNS) involvement may present as signs and symptoms
amplification of chromosome 21q (iAMP 21q) are associated of increased intracranial pressure, such as headaches and pap-
with a poor prognosis. illedema; or as cranial nerve palsies, nuchal rigidity, and rarely
Another aberration with a poor prognosis is the BCRF- hyperphagia and obesity caused by infiltration of the hypothala-
ABL fusion protein created by the t(9;22) “Philadelphia chromo- mus. Overt testicular leukemia manifests as painless testicular
some.” Its frequency is low in children (3–5%) and much higher enlargement. Mediastinal involvement, common in T-cell ALL,
in adults (at least 30%). The prognosis dramatically improved may cause dyspnea and superior vena cava syndrome.
by treatment with BCR–ABL1 inhibitors. Clinical laboratory findings often include anemia and throm-
The MLL(KMT2A) gene located on chromosome 11q23 is bocytopenia. Approximately 20% of children present with
involved in fusion translocations with >80 different partner genes. leukocyte counts >50 000/µL. Importantly, approximately 40%
The most common translocation in ALL fuses the MLL with the of children have leukocyte counts of <10 000/µL, and leukemic
AF4 gene on chromosome 4. It is characteristic of infant leukemia blasts may or may not be seen on peripheral smears. Therefore
and is associated with a poor prognosis. the diagnosis of leukemia may occasionally be missed in routine
The clinical significance of the E2A (TCF3)-PBX1 fusion automated blood count. Elevated serum lactate dehydrogenase
protein caused by t(1;19) translocation, occurring in <5% of activity and uric acid and phosphorous concentrations are
childhood ALL, has greatly improved by more intensive chemo- common in patients with a large leukemic cell burden.
therapy. The change in the prognostic significance of this particular The diagnosis of ALL is established by bone marrow examina-
translocation with improved therapy exemplifies the general tion. The normal bone marrow contains <5% blasts. A minimum
principle that the prognostic impact of a clinical or biological of 25% lymphoblasts on differential examination of the bone
parameter is highly dependent on the specific treatment protocol. marrow aspirate is necessary for the diagnosis of ALL. Most
E2A-PBX1 ALLs also represents a subtype of ALL with activation children with ALL have a hypercellular marrow with blasts
of signaling downstream to the preB-cell receptor (pre-BCR). constituting 60–100% of the nucleated cells.