Page 1138 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1138
1104 Part NiNe Transplantation
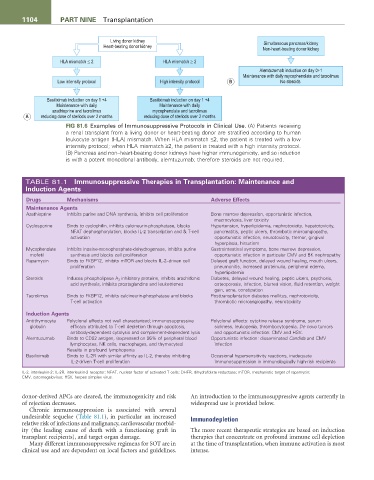
Living donor kidney Simultaneous pancreas/kidney
Heart-beating donor kidney
Non-heart-beating donor kidney
HLA mismatch £ 2 HLA mismatch ³ 2
Alemtuzumab induction on day 0+1
Maintenance with daily mycophenolate and tacrolimus
Low intensity protocol High intensity protocol B No steroids
Basiliximab induction on day 1 +4 Basiliximab induction on day 1 +4
Maintenance with daily Maintenance with daily
azathioprine and tacrolimus mycophenolate and tacrolimus
A reducing dose of steriods over 2 months reducing dose of steriods over 2 months
FiG 81.6 Examples of Immunosuppressive Protocols in Clinical Use. (A) Patients receiving
a renal transplant from a living donor or heart-beating donor are stratified according to human
leukocyte antigen (HLA) mismatch. When HLA mismatch ≤2, the patient is treated with a low
intensity protocol; when HLA mismatch ≥2, the patient is treated with a high intensity protocol.
(B) Pancreas and non–heart-beating donor kidneys have higher immunogenicity, and so induction
is with a potent monoclonal antibody, alemtuzumab; therefore steroids are not required.
TABLE 81.1 immunosuppressive therapies in transplantation: Maintenance and
induction agents
Drugs Mechanisms adverse effects
Maintenance agents
Azathioprine Inhibits purine and DNA synthesis, inhibits cell proliferation Bone marrow depression, opportunistic infection,
macrocytosis, liver toxicity
Cyclosporine Binds to cyclophilin, inhibits calcineurin-phosphatase, blocks Hypertension, hyperlipidemia, nephrotoxicity, hepatotoxicity,
NFAT dephosphorylation, blocks IL-2 transcription and & T-cell pancreatitis, peptic ulcers, thrombotic microangiopathy,
activation opportunistic infection, neurotoxicity, tremor, gingival
hyperplasia, hirsutism
Mycophenolate Inhibits inosine-monophosphate-dehydrogenase, inhibits purine Gastrointestinal symptoms, bone marrow depression,
mofetil synthesis and blocks cell proliferation opportunistic infection in particular CMV and BK nephropathy
Rapamycin Binds to FKBP12, inhibits mTOR and blocks IL-2–driven cell Delayed graft function, delayed wound healing, mouth ulcers,
proliferation pneumonitis, increased proteinuria, peripheral edema,
hyperlipidemia
Steroids Induces phospholipase A 2 inhibitory proteins, inhibits arachidonic Diabetes, delayed wound healing, peptic ulcers, psychosis,
acid synthesis, inhibits prostaglandins and leukotrienes osteoporosis, infection, blurred vision, fluid retention, weight
gain, acne, constipation
Tacrolimus Binds to FKBP12, inhibits calcineurin-phosphatase and blocks Posttransplantation diabetes mellitus, nephrotoxicity,
T-cell activation thrombotic microangiopathy, neurotoxicity
induction agents
Antithymocyte Polyclonal effects not well characterized; immunosuppressive Polyclonal effects: cytokine release syndrome, serum
globulin efficacy attributed to T-cell depletion through apoptosis, sickness, leukopenia, thrombocytopenia. De novo tumors
antibody-dependent cytolysis and complement-dependent lysis and opportunistic infection: CMV and HSV.
Alemtuzumab Binds to CD52 antigen, (expressed on 95% of peripheral blood Opportunistic infection: disseminated Candida and CMV
lymphocytes, NK cells, macrophages, and thymocytes) infection
Results in profound lymphopenia
Basiliximab Binds to IL-2R with similar affinity as IL-2, thereby inhibiting Occasional hypersensitivity reactions, inadequate
IL-2-driven T-cell proliferation immunosuppression in immunologically high-risk recipients
IL-2, interleukin-2; IL-2R, interleukin-2 receptor; NFAT, nuclear factor of activated T cells; DHFR, dihydrofolate reductase; mTOR, mechanistic target of rapamycin;
CMV, cytomegalovirus; HSV, herpes simplex virus.
donor-derived APCs are cleared, the immunogenicity and risk An introduction to the immunosuppressive agents currently in
of rejection decreases. widespread use is provided below.
Chronic immunosuppression is associated with several
undesirable sequelae (Table 81.1), in particular an increased Immunodepletion
relative risk of infections and malignancy, cardiovascular morbid-
ity (the leading cause of death with a functioning graft in The more recent therapeutic strategies are based on induction
transplant recipients), and target organ damage. therapies that concentrate on profound immune cell depletion
Many different immunosuppressive regimens for SOT are in at the time of transplantation, when immune activation is most
clinical use and are dependent on local factors and guidelines. intense.