Page 1224 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1224
CHAPTER 88 Protein Kinase Antagonists 1187
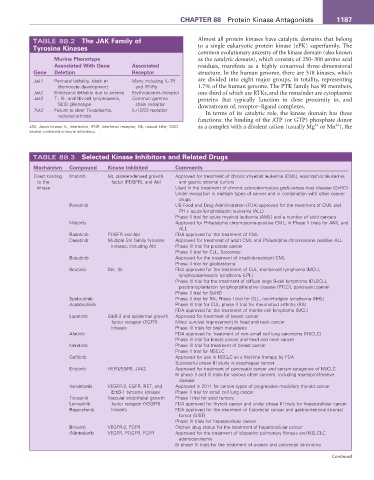
TABLE 88.2 The JAK Family of Almost all protein kinases have catalytic domains that belong
Tyrosine Kinases to a single eukaryotic protein kinase (ePK) superfamily. The
common evolutionary ancestry of the kinase domain (also known
Murine Phenotype as the catalytic domain), which consists of 250–300 amino acid
Associated With Gene Associated residues, manifests as a highly conserved three-dimensional
Gene Deletion Receptor structure. In the human genome, there are 518 kinases, which
Jak1 Perinatal lethality, block in Many including IL-7R are divided into eight major groups, in totality, representing
thymocyte development and IFNRs 1.7% of the human genome. The PTK family has 90 members,
Jak2 Embryonic lethality due to anemia Erythropoietin receptor one-third of which are RTKs, and the remainder are cytoplasmic
Jak3 T-, B-, and NK-cell lymphopenia, Common gamma proteins that typically function in close proximity to, and
SCID phenotype chain receptor downstream of, receptor–ligand complexes.
Tyk2 Failure to clear Toxoplasma, IL-12/23 receptor In terms of its catalytic role, the kinase domain has three
reduced arthritis
functions: the binding of the ATP (or GTP) phosphate donor
2+
2+
JAK, Janus kinase; IL, interleukin; IFNR, interferon receptor; NK, natural killer; SCID, as a complex with a divalent cation (usually Mg or Mn ), the
severe combined immune deficiency.
TABLE 88.3 Selected Kinase Inhibitors and Related Drugs
Mechanism Compound Kinase Inhibited Comments
Direct binding Imatinib Kit, platelet-derived growth Approved for treatment of chronic myeloid leukemia (CML), eosinophilic leukemia,
to the factor (PDGFR), and Abl and gastric stromal tumors
kinase Used in the treatment of chronic sclerodermatous graft-versus-host disease (GvHD)
Under evaluation in multiple types of cancer and in combination with other cancer
drugs
Ponatinib US Food and Drug Administration (FDA) approved for the treatment of CML and
PH + acute lymphoblastic leukemia (ALL)
Phase II trial for acute myeloid leukemia (AML) and a number of solid cancers
Nilotinib Approved for Philadelphia chromosome-positive CML; in Phase II trials for AML and
ALL
Radotinib PDGFR and Abl FDA approved for the treatment of CML
Dasatinib Multiple Src family tyrosine Approved for treatment of adult CML and Philadelphia chromosome positive ALL
kinases, including Abl Phase III trial for prostate cancer
Phase II trial for CLL, Sarcomas
Bosutinib Approved for the treatment of imatinib-resistant CML
Phase II trial for glioblastoma
Ibrutinib Btk, Itk FDA approved for the treatment of CLL, mantle-cell lymphoma (MCL),
lymphoplasmacytic lymphoma (LPL)
Phase III trial for the treatment of diffuse large B-cell lymphoma (DLBCL),
posttransplantation lymphoproliferative disease (PTLD), pancreatic cancer
Phase II trial for GvHD
Spebrutinib Phase II trial for RA, Phase I trial for CLL, non-Hodgkin lymphoma (NHL)
Acalabrutinib Phase III trial for CLL, phase II trial for rheumatoid arthritis (RA)
FDA approved for the treatment of mantle cell lymphoma (MCL)
Lapatinib ErbB-2 and epidermal growth Approved for treatment of breast cancer
factor receptor (EGFR) Minor survival improvement in head and neck cancer
kinases Phase III trials for brain metastasis
Afatinib FDA approved for treatment of non–small cell lung carcinoma (NSCLC)
Phase III trial for breast cancer and head and neck cancer
Neratinib Phase III trial for treatment of breast cancer
Phase II trial for NSCLC
Gefitinib Approved for use in NSCLC as a first-line therapy by FDA
Successful phase III study in esophageal cancer
Erlotinib HER1/EGFR, JAK2 Approved for treatment of pancreatic cancer and certain categories of NSCLC
In phase II and III trials for various other cancers, including myeloproliferative
disease
Vandetanib VEGFR-2, EGFR, RET, and Approved in 2011 for certain types of progressive medullary thyroid cancer
ErbB-1 tyrosine kinases Phase II trial for small cell lung cancer
Tivozanib Vascular endothelial growth Phase I trial for solid tumors
Lenvatinib factor receptor (VEGFR) FDA approved for thyroid cancer and under phase III trials for hepatocellular cancer
Regorafenib kinases FDA approved for the treatment of Colorectal cancer and gastrointestinal stromal
tumor (GIST)
Phase III trials for hepatocellular cancer
Brivanib VEGFR-2, FGFR Orphan drug status for the treatment of hepatocellular cancer
(N)intedanib VEGFR, PDGFR, FGFR Approved for the treatment of idiopathic pulmonary fibrosis and NSLCLC
adenocarcinoma
In phase III trials for the treatment of ovarian and colorectal carcinoma
Continued