Page 1227 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1227
1190 PART TEN Prevention and Therapy of Immunological Diseases
binding of the protein substrate, and the transfer of the Phosphorylated active
γ-phosphate from ATP or GTP to the protein substrate. Despite loop tyrosines
the huge number of serine/threonine and tyrosine kinases, there
is evidence of a common ancestor, and this is reflected in structural
similarities, particularly in the active (ATP bound) confirmation.
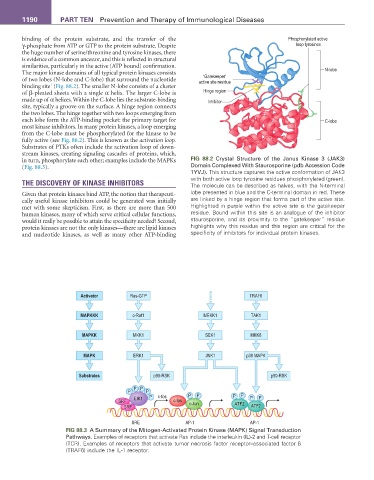
The major kinase domains of all typical protein kinases consists N-lobe
‘Gatekeeper’
of two lobes (N-lobe and C-lobe) that surround the nucleotide active site residue
1
binding site (Fig. 88.2). The smaller N-lobe consists of a cluster
of β-pleated sheets with a single α helix. The larger C-lobe is Hinge region
made up of α helices. Within the C-lobe lies the substrate-binding Inhibitor
site, typically a groove on the surface. A hinge region connects
the two lobes. The hinge together with two loops emerging from
each lobe form the ATP-binding pocket: the primary target for C-lobe
most kinase inhibitors. In many protein kinases, a loop emerging
from the C-lobe must be phosphorylated for the kinase to be
fully active (see Fig. 88.2). This is known as the activation loop.
Substrates of PTKs often include the activation loop of down-
stream kinases, creating signaling cascades of proteins, which,
in turn, phosphorylate each other; examples include the MAPKs FIG 88.2 Crystal Structure of the Janus Kinase 3 (JAK3)
(Fig. 88.3). Domain Complexed With Staurosporine (pdb Accession Code
1YVJ). This structure captures the active conformation of JAK3
with both active loop tyrosine residues phosphorylated (green).
THE DISCOVERY OF KINASE INHIBITORS The molecule can be described as halves, with the N-terminal
Given that protein kinases bind ATP, the notion that therapeuti- lobe presented in blue and the C-terminal domain in red. These
cally useful kinase inhibitors could be generated was initially are linked by a hinge region that forms part of the active site.
met with some skepticism. First, as there are more than 500 Highlighted in purple within the active site is the gatekeeper
human kinases, many of which serve critical cellular functions, residue. Bound within this site is an analogue of the inhibitor
would it really be possible to attain the specificity needed? Second, staurosporine, and its proximity to the “gatekeeper” residue
protein kinases are not the only kinases—there are lipid kinases highlights why this residue and this region are critical for the
and nucleotide kinases, as well as many other ATP-binding specificity of inhibitors for individual protein kinases.
Activator Ras-GTP TRAF6
MAPKKK c-Raf1 MEKK1 TAK1
MAPKK MKK1 SEK1 MKK6
MAPK ERK1 JNK1 p38 MAPK
Substrates p90-RSK p90-RSK
P P
P P
EIK1 P c-fos P P P P P P
SRF c-fos c-Jun ATF2
SRF ATF2
SRE AP-1 AP-1
FIG 88.3 A Summary of the Mitogen-Activated Protein Kinase (MAPK) Signal Transduction
Pathways. Examples of receptors that activate Ras include the interleukin (IL)-2 and T-cell receptor
(TCR). Examples of receptors that activate tumor necrosis factor receptor–associated factor 6
(TRAF6) include the IL-1 receptor.