Page 1225 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1225
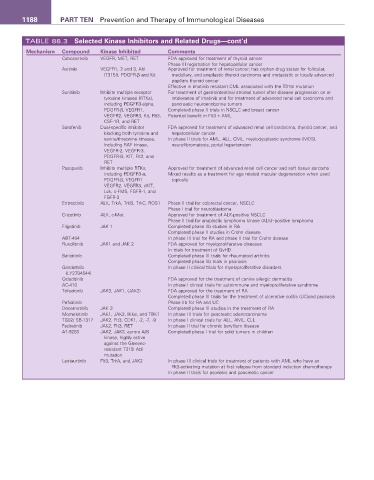
1188 PART TEN Prevention and Therapy of Immunological Diseases
TABLE 88.3 Selected Kinase Inhibitors and Related Drugs—cont’d
Mechanism Compound Kinase Inhibited Comments
Cabozantinib VEGFR, MET, RET FDA approved for treatment of thyroid cancer
Phase III registration for hepatocellular cancer
Axitinib VEGFR1, 2 and 3, Abl Approved for treatment of renal cancer; has orphan drug status for follicular,
(T315I), PDGFR-β and Kit medullary, and anaplastic thyroid carcinoma and metastatic or locally advanced
papillary thyroid cancer
Effective in imatinib resistant CML associated with the T315I mutation
Sunitinib Inhibits multiple receptor For treatment of gastrointestinal stromal tumor after disease progression on or
tyrosine kinases (RTKs), intolerance of imatinib and for treatment of advanced renal cell carcinoma and
including PDGFR3-alpha, pancreatic neuroendocrine tumors
PDGFR-β, VEGFR1, Completed phase II trials in NSCLC and breast cancer
VEGFR2, VEGFR3, Kit, Flt3, Potential benefit in Flt3 + AML
CSF-1R, and RET
Sorafenib Dual-specific inhibitor FDA approved for treatment of advanced renal cell carcinoma, thyroid cancer, and
blocking both tyrosine and hepatocellular cancer
serine/threonine kinases, In phase II trials for AML, ALL, CML, myelodysplastic syndrome (MDS),
including RAF kinase, neurofibromatosis, portal hypertension
VEGFR-2, VEGFR-3,
PDGFR-B, KIT, Flt3, and
RET
Pazopanib Inhibits multiple RTKs, Approved for treatment of advanced renal cell cancer and soft tissue sarcoma
including PDGFR3-α, Mixed results as a treatment for age related macular degeneration when used
PDGFR-β, VEGFR1 topically
VEGFR2, VEGFR3, cKIT,
Lck, c-FMS, FGFR-1, and
FGFR-3
Entrectinib ALK, TrkA, TrkB, TrkC, ROS1 Phase II trial for colorectal cancer, NSCLC
Phase I trial for neuroblastoma
Crizotinib ALK, c-Met Approved for treatment of ALK-positive NSCLC
Phase II trial for anaplastic lymphoma kinase (ALK)–positive lymphoma
Filgotinib JAK 1 Completed phase IIb studies in RA
Completed phase II studies in Crohn disease
ABT-494 In phase III trial for RA and phase II trial for Crohn disease
Ruxolitinib JAK1 and JAK 2 FDA approved for myeloproliferative diseases
In trials for treatment of GvHD
Baricitinib Completed phase III trials for rheumatoid arthritis
Completed phase IIb trials in psoriasis
Gandotinib In phase II clinical trials for myeloproliferative disorders
(LY2784544)
Oclacitinib FDA approved for the treatment of canine allergic dermatitis
AC-410 In phase I clinical trials for autoimmune and myeloproliferative syndrome
Tofacitinib JAK3, JAK1, (JAK2) FDA approved for the treatment of RA
Completed phase III trials for the treatment of ulcerative colitis (UC)and psoriasis
Peficitinib Phase IIb for RA and UC
Decernotinib JAK 3 Completed phase III studies in the treatment of RA
Momelotinib JAK1, JAK2, IKKe, and TBK1 In phase III trials for pancreatic adenocarcinoma
TG02/ SB-1317 JAK2, Flt3, CDK1, -2, -7, -9 In phase I clinical trials for ALL, AML, CLL
Fedratinib JAK2, Flt3, RET In phase II trial for chronic beryllium disease
AT-9283 JAK2, JAK3, aurora A/B Completed phase I trial for solid tumors in children
kinase, highly active
against the Gleevec-
resistant T315l Abl
mutation
Lestaurtinib Flt3, TrkA, and JAK2 In phase III clinical trials for treatment of patients with AML who have an
Flt3-activating mutation at first relapse from standard induction chemotherapy
In phase II trials for psoriasis and pancreatic cancer