Page 1226 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 1226
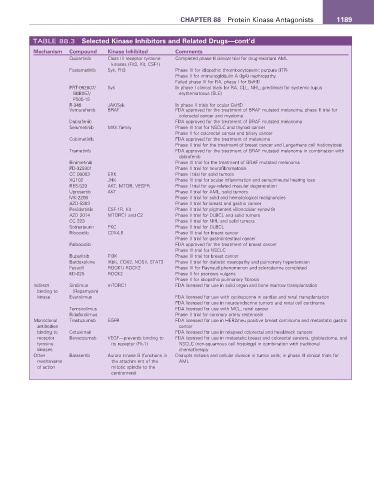
CHAPTER 88 Protein Kinase Antagonists 1189
TABLE 88.3 Selected Kinase Inhibitors and Related Drugs—cont’d
Mechanism Compound Kinase Inhibited Comments
Quizartinib Class III receptor tyrosine Completed phase II clinical trial for drug-resistant AML
kinases (Flt3, Kit, CSF1)
Fostamatinib Syk, Flt3 Phase III for idiopathic thrombocytopenic purpura (ITP)
Phase II for immunoglobulin A (IgA) nephropathy
Failed phase III for RA, phase I for GvHD
PRT-062607/ Syk In phase I clinical trials for RA, CLL, NHL; preclinical for systemic lupus
BIIB057/ erythematosus (SLE)
P505-15
R-348 JAK/Syk In phase II trials for ocular GvHD
Vemurafenib BRAF FDA approved for the treatment of BRAF mutated melanoma; phase II trial for
colorectal cancer and myeloma
Dabrafenib FDA approved for the treatment of BRAF mutated melanoma
Selumetinib MKK family Phase III trial for NSCLC and thyroid cancer
Phase II for colorectal cancer and biliary cancer
Cobimetinib FDA approved for the treatment of melanoma
Phase II trial for the treatment of breast cancer and Langerhans cell histiocytosis
Trametinib FDA approved for the treatment of BRAF mutated melanoma in combination with
dabrafenib
Binimetinib Phase III trial for the treatment of BRAF mutated melanoma
PD-325901 Phase II trial for neurofibromatosis
CC 90003 ERK Phase I trial for solid tumors
XG102 JNK Phase III trial for ocular inflammation and sensorineural hearing loss
RES-529 AKT, MTOR, VEGFR Phase I trial for age-related macular degeneration
Uprosertib AKT Phase II trial for AML, solid tumors
MK-2206 Phase II trial for solid and hematological malignancies
AZD-5363 Phase II trial for breast and gastric cancer
Pexidartinib CSF-1R, Kit Phase II trial for pigmented villonodular synovitis
AZD 2014 MTORC1 and C2 Phase II trial for DLBCL and solid tumors
CC 223 Phase II trial for NHL and solid tumors
Sotrastaurin PKC Phase II trial for DLBCL
Ribociclib CDK4,6 Phase III trial for breast cancer
Phase II trial for gastrointestinal cancer
Palbociclib FDA approved for the treatment of breast cancer
Phase III trial for NSCLC
Buparlisib PI3K Phase III trial for breast cancer
Bardoxolone IKbK, COX2, NOSII, STAT3 Phase II trial for diabetic neuropathy and pulmonary hypertension
Fasudil ROCK1/ ROCK2 Phase III for Raynaud phenomenon and scleroderma completed
KD-025 ROCK2 Phase II for psoriasis vulgaris
Phase II for idiopathic pulmonary fibrosis
Indirect Sirolimus mTORC1 FDA licensed for use in solid organ and bone marrow transplantation
binding to (Rapamycin)
kinase Everolimus FDA licensed for use with cyclosporine in cardiac and renal transplantation
FDA licensed for use in neuroendocrine tumors and renal cell carcinoma
Temsirolimus FDA licensed for use with MCL, renal cancer
Ridaforolimus Phase II trial for coronary artery restenosis
Monoclonal Trastuzumab EGFR FDA licensed for use in HER2/neu positive breast carcinoma and metastatic gastric
antibodies cancer
binding to Cetuximab FDA licensed for use in relapsed colorectal and head/neck cancers
receptor Bevacizumab VEGF—prevents binding to FDA licensed for use in metastatic breast and colorectal cancers, glioblastoma, and
tyrosine its receptor (Flt-1) NSCLC (non-squamous cell histology) in combination with traditional
kinases chemotherapy
Other Barasertib Aurora kinase B (functions in Disrupts mitosis and cellular division in tumor cells; in phase III clinical trials for
mechanisms the attachm ent of the AML
of action mitotic spindle to the
centromere)