Page 194 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 194
CHaPTEr 11 Lymphocyte Adhesion and Trafficking 175
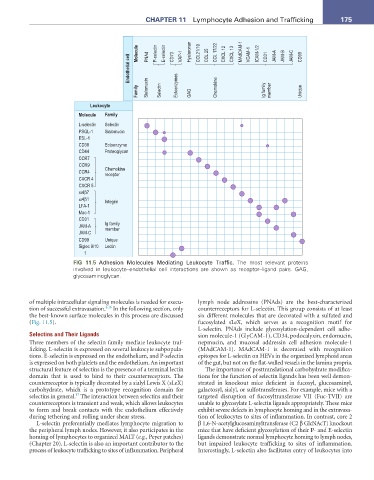
Molecule PNAd P-selectin E-selectin CD73 VAP-1 Hyaluronan CCL21/19 CCL 25 CCL 17/22 CXCL 12 CXCL 13 MAdCAM-1 VCAM-1 ICAM-1/2 CD31 JAM-A JAM-B JAM-C CD99
Endothelial cell
Family Sialomucin Selectin Ectoenzymes GAG Chemokine Ig family member Unique
Leukocyte
Molecule Family
L-selectin Selectin
PSGL-1 Sialomucin
ESL-1
CD38 Ectoenzyme
CD44 Proteoglycan
CCR7
CCR9
CCR4 Chemokine
receptor
CXCR 4
CXCR 5
α4β7
α4β1 Integrin
LFA-1
Mac-1
CD31
JAM-A Ig family
member
JAM-C
CD99 Unique
Siglec 9/10 Lectin
?
FIG 11.5 Adhesion Molecules Mediating Leukocyte Traffic. The most relevant proteins
involved in leukocyte–endothelial cell interactions are shown as receptor–ligand pairs. GAG,
glycosaminoglycan.
of multiple intracellular signaling molecules is needed for execu- lymph node addressins (PNAds) are the best-characterized
2,16
tion of successful extravasation. In the following section, only counterreceptors for L-selectin. This group consists of at least
the best-known surface molecules in this process are discussed six different molecules that are decorated with a sulfated and
(Fig. 11.5). fucosylated sLeX, which serves as a recognition motif for
L-selectin. PNAds include glycosylation-dependent cell adhe-
Selectins and Their Ligands sion molecule-1 (GlyCAM-1), CD34, podocalyxin, endomucin,
Three members of the selectin family mediate leukocyte traf- nepmucin, and mucosal addressin cell adhesion molecule-1
ficking. L-selectin is expressed on several leukocyte subpopula- (MAdCAM-1). MAdCAM-1 is decorated with recognition
tions. E-selectin is expressed on the endothelium, and P-selectin epitopes for L-selectin on HEVs in the organized lymphoid areas
is expressed on both platelets and the endothelium. An important of the gut, but not on the flat-walled vessels in the lamina propria.
structural feature of selectins is the presence of a terminal lectin The importance of posttranslational carbohydrate modifica-
domain that is used to bind to their counterreceptors. The tions for the function of selectin ligands has been well demon-
counterreceptor is typically decorated by a sialyl Lewis X (sLeX) strated in knockout mice deficient in fucosyl, glucosaminyl,
carbohydrate, which is a prototype recognition domain for galactosyl, sialyl, or sulfotransferases. For example, mice with a
17
selectins in general. The interaction between selectins and their targeted disruption of fucosyltransferase VII (Fuc-TVII) are
counterreceptors is transient and weak, which allows leukocytes unable to glycosylate L-selectin ligands appropriately. These mice
to form and break contacts with the endothelium effectively exhibit severe defects in lymphocyte homing and in the extravasa-
during tethering and rolling under shear stress. tion of leukocytes to sites of inflammation. In contrast, core 2
L-selectin preferentially mediates lymphocyte migration to β 1,6-N-acetylglucosaminyltransferase (C2 β GlcNAcT) knockout
the peripheral lymph nodes. However, it also participates in the mice that have deficient glycosylation of their P- and E-selectin
homing of lymphocytes to organized MALT (e.g., Peyer patches) ligands demonstrate normal lymphocyte homing to lymph nodes,
(Chapter 20). L-selectin is also an important contributor to the but impaired leukocyte trafficking to sites of inflammation.
process of leukocyte trafficking to sites of inflammation. Peripheral Interestingly, L-selectin also facilitates entry of leukocytes into