Page 205 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 205
CHAPTER 12 T-Cell Activation and Tolerance 185
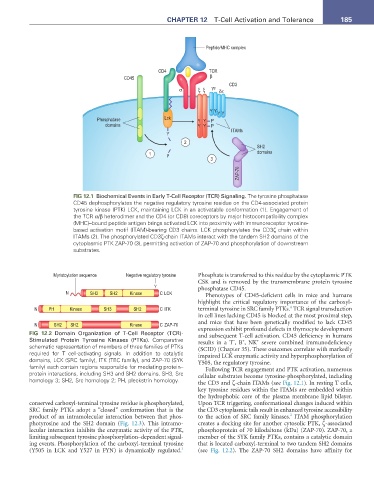
Peptide/MHC complex
CD4 TCR
CD45 β
CD3
α ξξ γε δε
YY YY
Phosphatase Lck Y Y P
domains Y Y P
ITAMs
Y
2
SH2
1 P domains
3
ZAP-70
FIG 12.1 Biochemical Events in Early T-Cell Receptor (TCR) Signaling. The tyrosine phosphatase
CD45 dephosphorylates the negative regulatory tyrosine residue on the CD4-associated protein
tyrosine kinase (PTK) LCK, maintaining LCK in an activatable conformation (1). Engagement of
the TCR α/β heterodimer and the CD4 (or CD8) coreceptors by major histocompatibility complex
(MHC)–bound peptide antigen brings activated LCK into proximity with immunoreceptor tyrosine-
based activation motif (ITAM)-bearing CD3 chains. LCK phosphorylates the CD3ζ chain within
ITAMs (2). The phosphorylated CD3ζ-chain ITAMs interact with the tandem SH2 domains of the
cytoplasmic PTK ZAP-70 (3), permitting activation of ZAP-70 and phosphorylation of downstream
substrates.
Myristoylation sequence Negative regulatory tyrosine Phosphate is transferred to this residue by the cytoplasmic PTK
CSK and is removed by the transmembrane protein tyrosine
Y phosphatase CD45.
N SH3 SH2 Kinase C LCK Phenotypes of CD45-deficient cells in mice and humans
highlight the critical regulatory importance of the carboxyl-
4
N PH Kinase SH3 SH2 C ITK terminal tyrosine in SRC family PTKs. TCR signal transduction
in cell lines lacking CD45 is blocked at the most proximal step,
N SH2 SH2 Kinase C ZAP-70 and mice that have been genetically modified to lack CD45
FIG 12.2 Domain Organization of T-Cell Receptor (TCR)- expression exhibit profound defects in thymocyte development
and subsequent T-cell activation. CD45 deficiency in humans
Stimulated Protein Tyrosine Kinases (PTKs). Comparative results in a T , B , NK severe combined immunodeficiency
−
+
+
schematic representation of members of three families of PTKs (SCID) (Chapter 35). These outcomes correlate with markedly
required for T cell-activating signals. In addition to catalytic impaired LCK enzymatic activity and hyperphosphorylation of
domains, LCK (SRC family), ITK (TEC family), and ZAP-70 (SYK Y505, the regulatory tyrosine.
family) each contain regions responsible for mediating protein– Following TCR engagement and PTK activation, numerous
protein interactions, including SH3 and SH2 domains. SH3, Src cellular substrates become tyrosine-phosphorylated, including
homology 3; SH2, Src homology 2; PH, pleckstrin homology. the CD3 and ζ-chain ITAMs (see Fig. 12.1). In resting T cells,
key tyrosine residues within the ITAMs are embedded within
the hydrophobic core of the plasma membrane lipid bilayer.
conserved carboxyl-terminal tyrosine residue is phosphorylated, Upon TCR triggering, conformational changes induced within
SRC family PTKs adopt a “closed” conformation that is the the CD3 cytoplasmic tails result in enhanced tyrosine accessibility
2
product of an intramolecular interaction between that phos- to the action of SRC family kinases. ITAM phosphorylation
photyrosine and the SH2 domain (Fig. 12.3). This intramo- creates a docking site for another cytosolic PTK, ζ-associated
lecular interaction inhibits the enzymatic activity of the PTK, phosphoprotein of 70 kilodaltons (kDa) (ZAP-70). ZAP-70, a
limiting subsequent tyrosine phosphorylation–dependent signal- member of the SYK family PTKs, contains a catalytic domain
ing events. Phosphorylation of the carboxyl-terminal tyrosine that is located carboxyl-terminal to two tandem SH2 domains
3
(Y505 in LCK and Y527 in FYN) is dynamically regulated. (see Fig. 12.2). The ZAP-70 SH2 domains have affinity for