Page 297 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 297
278 ParT TwO Host Defense Mechanisms and Inflammation
A B
C
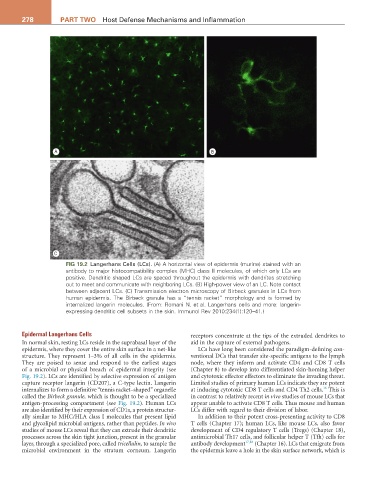
FIG 19.2 Langerhans Cells (LCs). (A) A horizontal view of epidermis (murine) stained with an
antibody to major histocompatibility complex (MHC) class II molecules, of which only LCs are
positive. Dendritic shaped LCs are spaced throughout the epidermis with dendrites stretching
out to meet and communicate with neighboring LCs. (B) High-power view of an LC. Note contact
between adjacent LCs. (C) Transmission electron microscopy of Birbeck granules in LCs from
human epidermis. The Birbeck granule has a “tennis racket” morphology and is formed by
internalized langerin molecules. (From: Romani N. et al. Langerhans cells and more: langerin-
expressing dendritic cell subsets in the skin. Immunol Rev 2010:234(1):120–41.)
Epidermal Langerhans Cells receptors concentrate at the tips of the extruded dendrites to
In normal skin, resting LCs reside in the suprabasal layer of the aid in the capture of external pathogens.
epidermis, where they cover the entire skin surface in a net-like LCs have long been considered the paradigm-defining con-
structure. They represent 1–3% of all cells in the epidermis. ventional DCs that transfer site-specific antigens to the lymph
They are poised to sense and respond to the earliest stages node, where they inform and activate CD4 and CD8 T cells
of a microbial or physical breach of epidermal integrity (see (Chapter 8) to develop into differentiated skin-homing helper
Fig. 19.2). LCs are identified by selective expression of antigen and cytotoxic effector effectors to eliminate the invading threat.
capture receptor langerin (CD207), a C-type lectin. Langerin Limited studies of primary human LCs indicate they are potent
16
internalizes to form a definitive “tennis racket–shaped” organelle at inducing cytotoxic CD8 T cells and CD4 Th2 cells. This is
called the Birbeck granule, which is thought to be a specialized in contrast to relatively recent in vivo studies of mouse LCs that
antigen-processing compartment (see Fig. 19.2). Human LCs appear unable to activate CD8 T cells. Thus mouse and human
are also identified by their expression of CD1a, a protein structur- LCs differ with regard to their division of labor.
ally similar to MHC/HLA class I molecules that present lipid In addition to their potent cross-presenting activity to CD8
and glycolipid microbial antigens, rather than peptides. In vivo T cells (Chapter 17); human LCs, like mouse LCs, also favor
studies of mouse LCs reveal that they can extrude their dendritic development of CD4 regulatory T cells (Tregs) (Chapter 18),
processes across the skin tight junction, present in the granular antimicrobial Th17 cells, and follicular helper T (Tfh) cells for
layer, through a specialized pore, called tricellulin, to sample the antibody development 17,18 (Chapter 16). LCs that emigrate from
microbial environment in the stratum corneum. Langerin the epidermis leave a hole in the skin surface network, which is