Page 538 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 538
CHaPTEr 36 Immunodeficiencies at the Interface of Innate and Adaptive Immunity 517
cells differentiated in vitro from patient-specific induced plu-
ripotent stem cells displayed uncontrolled virus replication and
impaired IFN-β production. The patient had no other serious
viral infections despite being seropositive for a myriad respiratory
10
viruses and is currently healthy at the age of 8 years. Thus the
IRF7-dependent IFN amplification pathway is crucial for the
mounting of antiviral responses to influenza virus but may be
redundant for other viruses.
GENETIC DISORDERS OF
NF-κB–MEDIATED IMMUNITY A
This group of inherited disorders leads to impaired NF-κB signal-
ing and strong susceptibility to pyogenic bacteria. Affected patients
bear mutations of NEMO, NFKBIA, IRAK4, MYD88, HOIL1, or
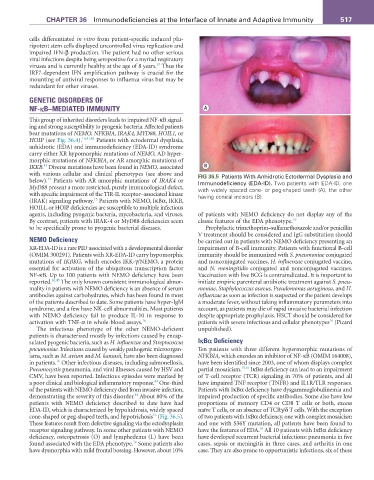
HOIP (see Fig. 36.4). 16,41,42 Patients with ectodermal dysplasia,
anhidrotic (EDA) and immunodeficiency (EDA-ID) syndrome
carry either XR hypomorphic mutations of NEMO, AD hyper-
morphic mutations of NFKBIA, or AR amorphic mutations of
43
IKKB. Diverse mutations have been found in NEMO, associated B
with various cellular and clinical phenotypes (see above and fIG 36.5 Patients With Anhidrotic Ectodermal Dysplasia and
44
below). Patients with AR amorphic mutations of IRAK4 or Immunodeficiency (EDA-ID). Two patients with EDA-ID, one
MyD88 present a more restricted, purely immunological defect, with widely spaced cone- or peg-shaped teeth (A), the other
with specific impairment of the TIR-IL receptor–associated kinase having conical incisors (B).
13
(IRAK) signaling pathway. Patients with NEMO, IκBα, IKKB,
HOIL1, or HOIP deficiencies are susceptible to multiple infectious
agents, including pyogenic bacteria, mycobacteria, and viruses. of patients with NEMO deficiency do not display any of the
By contrast, patients with IRAK-4 or MyD88 deficiencies seem classic features of the EDA phenotype. 16
to be specifically prone to pyogenic bacterial diseases. Prophylactic trimethoprim–sulfamethoxazole and/or penicillin
V treatment should be considered and IgG substitution should
NEMO Deficiency be carried out in patients with NEMO deficiency presenting an
XR-EDA-ID is a rare PID associated with a developmental disorder impairment of B-cell immunity. Patients with functional B-cell
(OMIM 300291). Patients with XR-EDA-ID carry hypomorphic immunity should be immunized with S. pneumoniae conjugated
mutations of IKBKG, which encodes IKK-γ/NEMO, a protein and nonconjugated vaccines, H. influenzae conjugated vaccine,
essential for activation of the ubiquitous transcription factor and N. meningitidis conjugated and nonconjugated vaccines.
NF-κB. Up to 100 patients with NEMO deficiency have been Vaccination with live BCG is contraindicated. It is important to
reported. 16,43 The only known consistent immunological abnor- initiate empiric parenteral antibiotic treatment against S. pneu-
mality in patients with NEMO deficiency is an absence of serum moniae, Staphylococcus aureus, Pseudomonas aeruginosa, and H.
antibodies against carbohydrates, which has been found in most influenzae as soon as infection is suspected or the patient develops
of the patients described to date. Some patients have hyper-IgM a moderate fever, without taking inflammatory parameters into
syndrome, and a few have NK-cell abnormalities. Most patients account, as patients may die of rapid invasive bacterial infection
with NEMO deficiency fail to produce IL-10 in response to despite appropriate prophylaxis. HSCT should be considered for
16
activation with TNF-α in whole-blood assays. 16 patients with severe infectious and cellular phenotypes (Picard
The infectious phenotype of the other NEMO-deficient unpublished).
patients is characterized mostly by infections caused by encap-
sulated pyogenic bacteria, such as H. influenzae and Streptococcus IκBα Deficiency
pneumoniae. Infections caused by weakly pathogenic microorgan- Ten patients with three different hypermorphic mutations of
isms, such as M. avium and M. kansasii, have also been diagnosed NFKBIA, which encodes an inhibitor of NF-κB (OMIM 164008),
16
in patients. Other infectious diseases, including salmonellosis, have been identified since 2003, one of whom displays complex
Pneumocystis pneumonia, and viral illnesses caused by HSV and partial mosaicism. 15,16 IκBα deficiency can lead to an impairment
CMV, have been reported. Infectious episodes were marked by of T-cell receptor (TCR) signaling in 70% of patients, and all
44
a poor clinical and biological inflammatory response. One-third have impaired TNF receptor (TNFR) and IL1R/TLR responses.
of the patients with NEMO deficiency died from invasive infection, Patients with IκBα deficiency have dysgammaglobulinemia and
44
demonstrating the severity of this disorder. About 80% of the impaired production of specific antibodies. Some also have low
patients with NEMO deficiency described to date have had proportions of memory CD4 or CD8 T cells or both, excess
EDA-ID, which is characterized by hypohidrosis, widely spaced naïve T cells, or an absence of TCRγ/δ T cells. With the exception
16
cone-shaped or peg-shaped teeth, and hypotrichosis (Fig. 36.5). of two patients with IκBα deficiency, one with complex mosaicism
These features result from defective signaling via the ectodysplasin and one with S36Y mutation, all patients have been found to
16
receptor signaling pathway. In some other patients with NEMO have the features of EDA. All 10 patients with IκBα deficiency
deficiency, osteopetrosis (O) and lymphedema (L) have been have developed recurrent bacterial infections: pneumonia in five
16
found associated with the EDA phenotype. Some patients also cases, sepsis or meningitis in three cases, and arthritis in one
have dysmorphia with mild frontal bossing. However, about 10% case. They are also prone to opportunistic infections, six of these