Page 536 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 536
CHaPTEr 36 Immunodeficiencies at the Interface of Innate and Adaptive Immunity 515
TLR-1, 2, 5, 6, 10 IL-1Rs
RANK/ TLR-4
VEGFR3/ TNFR-S
EDAR TCR/BCR
MD2
TLR-7,
8, 9
MyD88
TRIF
TLR-3
UNC-93B
TRAF3
IRAK4 UNC-93B
IRAK1 IKK
NEMO TBK1
IKK
αβ
p p Iκ Bα
Iκ Bα
Iκ Bα Iκ Bα MAPK IRF3
κ
B
α
I
Iκ Bα
NF-κB p50 p65 p50 p65
p50 p65 AP1 IRF3
IL-6, IL-1, TNFα IL-6, IL-1, TNFα IFNβ
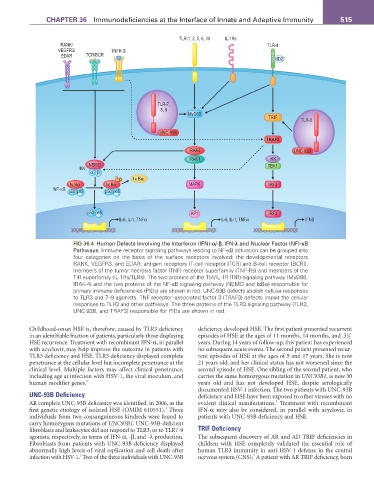
fIG 36.4 Human Defects Involving the Interferon (IFN)-α/-β, IFN-λ and Nuclear Factor (NF)-κB
Pathways. Immune receptor signaling pathways leading to NF-κB activation can be grouped into
four categories on the basis of the surface receptors involved: the developmental receptors
RANK, VEGFR3, and EDAR; antigen receptors (T-cell receptor [TCR] and B-cell receptor [BCR]);
members of the tumor necrosis factor (TNF) receptor superfamily (TNF-Rs) and members of the
TIR superfamily (IL-1Rs/TLRs). The two proteins of the Toll/IL-1R (TIR) signaling pathway (MyD88,
IRAK-4) and the two proteins of the NF-κB signaling pathway (NEMO and IκBα) responsible for
primary immune deficiencies (PIDs) are shown in red. UNC-93B defects abolish cellular responses
to TLR3 and 7–9 agonists. TNF receptor–associated factor 3 (TRAF3) defects impair the cellular
responses to TLR3 and other pathways. The three proteins of the TLR3 signaling pathway (TLR3,
UNC-93B, and TRAF3) responsible for PIDs are shown in red.
Childhood-onset HSE is, therefore, caused by TLR3 deficiency deficiency developed HSE. The first patient presented recurrent
in an identifiable fraction of patients, particularly those displaying episodes of HSE at the ages of 11 months, 14 months, and 3 2
1
HSE recurrence. Treatment with recombinant IFN-α, in parallel years. During 14 years of follow-up, this patient has experienced
with acyclovir, may help improve the outcome in patients with no subsequent acute events. The second patient presented recur-
TLR3 deficiency and HSE. TLR3 deficiency displayed complete rent episodes of HSE at the ages of 5 and 17 years. She is now
penetrance at the cellular level but incomplete penetrance at the 21 years old, and her clinical status has not worsened since the
clinical level. Multiple factors may affect clinical penetrance, second episode of HSE. One sibling of the second patient, who
including age at infection with HSV-1, the viral inoculum, and carries the same homozygous mutation in UNC93B1, is now 30
human modifier genes. 8 years old and has not developed HSE, despite serologically
documented HSV-1 infection. The two patients with UNC-93B
UNC-93B Deficiency deficiency and HSE have been exposed to other viruses with no
3
AR complete UNC-93B deficiency was identified, in 2006, as the evident clinical manifestations. Treatment with recombinant
3
first genetic etiology of isolated HSE (OMIM 610551). Three IFN-α may also be considered, in parallel with acyclovir, in
individuals from two consanguineous kindreds were found to patients with UNC-93B deficiency and HSE.
carry homozygous mutations of UNC93B1. UNC-93B-deficient
fibroblasts and leukocytes did not respond to TLR3, or to TLR7-9 TRIF Deficiency
agonists, respectively, in terms of IFN-α, -β, and -λ production. The subsequent discovery of AR and AD TRIF deficiencies in
Fibroblasts from patients with UNC-93B deficiency displayed children with HSE completely validated the essential role of
abnormally high levels of viral replication and cell death after human TLR3 immunity in anti-HSV-1 defense in the central
3
7
infection with HSV-1. Two of the three individuals with UNC-93B nervous system (CNS). A patient with AR TRIF deficiency, born