Page 802 - Clinical Immunology_ Principles and Practice ( PDFDrive )
P. 802
CHaPtEr 57 Spondyloarthritis 773
%
% ( % ($ % H[RQ % H[RQ % %
1$ 6($ $IU 6DUG ($ (8 1$
7XU 6($ 8. ($ (8
1$ 1$
7XU ($ 1$ 0( (8 (8 (8
(8 ($ 0H[ 1$ $IU
6($ (8 (8 ($ (8
($ (8 1$ 1$
($ (8 1($ (8 ($
6$ ($ (8 ($
($ 7XU (8 ($
($ ($ (8 ($
($ (8 6($
($ (8
($ (8 (8
($ (8
6($ ($ (8
(8
(8
(8
(8
(8
6($
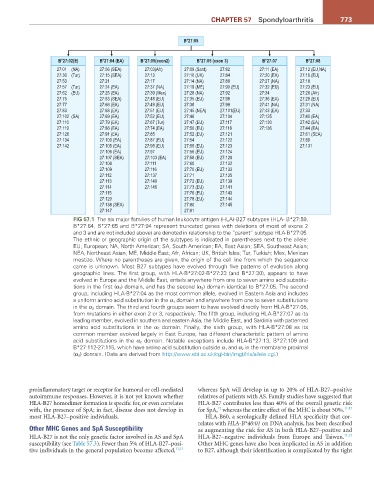
FiG 57.1 The six major families of human leukocyte antigen (HLA)-B27 subtypes (HLA- B*27:59,
B*27:64, B*27:65 and B*27:94 represent truncated genes with deletions of most of exons 2
and 3 and are not included above) are denoted in relationship to the “parent” subtype HLA-B*27:05.
The ethnic or geographic origin of the subtypes is indicated in parentheses next to the allele:
EU, European; NA, North American; SA, South American; EA, East Asian; SEA, Southeast Asian;
NEA, Northeast Asian; ME, Middle East; Afr, African; UK, British Isles; Tur, Turkish; Mex, Mexican
mestizo. Where no parentheses are given, the origin of the cell line from which the sequence
came is unknown. Most B27 subtypes have evolved through five patterns of evolution along
geographic lines. The first group, with HLA-B*27:02-B*27:23 (and B*27:30), appears to have
evolved in Europe and the Middle East, entails anywhere from one to seven amino acid substitu-
tions in the first (α 1 ) domain, and has the second (α 2 ) domain identical to B*27:05. The second
group, including HLA-B*27:04 as the most common allele, evolved in Eastern Asia and includes
a uniform amino acid substitution in the α 1 domain and anywhere from one to seven substitutions
in the α 2 domain. The third and fourth groups seem to have evolved directly from HLA-B*27:05,
from mutations in either exon 2 or 3, respectively. The fifth group, including HLA-B*27:07 as its
leading member, evolved in southern and eastern Asia, the Middle East, and Sardinia with patterned
amino acid substitutions in the α 2 domain. Finally, the sixth group, with HLA-B*27:08 as its
common member evolved largely in East Europe, has different characteristic pattern of amino
acid substitutions in the α 2 domain. Notable exceptions include HLA-B*27:13, B*27:109 and
B*27:112-27:115, which have amino acid substitution outside α 1 and α 2 in the membrane proximal
(α 3 ) domain. (Data are derived from http://www.ebi.ac.uk/cgi-bin/imgt/hla/allele.cgi.)
proinflammatory target or receptor for humoral or cell-mediated whereas SpA will develop in up to 20% of HLA-B27–positive
autoimmune responses. However, it is not yet known whether relatives of patients with AS. Family studies have suggested that
HLA-B27 homodimer formation is specific for, or even correlates HLA-B27 contributes less than 40% of the overall genetic risk
12
with, the presence of SpA; in fact, disease does not develop in for SpA, whereas the entire effect of the MHC is about 50%. 11,13
most HLA-B27–positive individuals. HLA-B60, a serologically defined HLA specificity that cor-
relates with HLA-B*40:01 on DNA analysis, has been described
Other MHC Genes and SpA Susceptibility as augmenting the risk for AS in both HLA-B27–positive and
HLA-B27 is not the only genetic factor involved in AS and SpA HLA-B27–negative individuals from Europe and Taiwan. 11,13
susceptibility (see Table 57.3). Fewer than 5% of HLA-B27–posi- Other MHC genes have also been implicated in AS in addition
tive individuals in the general population become affected, 11,13 to B27, although their identification is complicated by the tight