Page 689 - Clinical Hematology_ Theory _ Procedures ( PDFDrive )
P. 689
CHAPTER 32 ■ Laboratory Manual: Manual Procedures in Hematology 673
HEMATOLOGY PROCEDURES (continued)
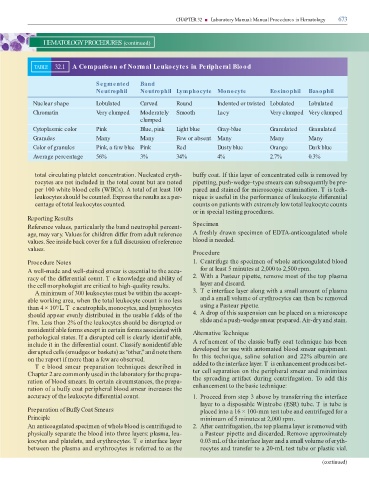
TABLE 32.1 A Comparison of Normal Leukocytes in Peripheral Blood
Segmented Band
Neutrophil Neutrophil Lymphocyte Monocyte Eosinophil Basophil
Nuclear shape Lobulated Curved Round Indented or twisted Lobulated Lobulated
Chromatin Very clumped Moderately Smooth Lacy Very clumped Very clumped
clumped
Cytoplasmic color Pink Blue, pink Light blue Gray-blue Granulated Granulated
Granules Many Many Few or absent Many Many Many
Color of granules Pink, a few blue Pink Red Dusty blue Orange Dark blue
Average percentage 56% 3% 34% 4% 2.7% 0.3%
total circulating platelet concentration. Nucleate eryth- bu y coat. I this layer o concentrate cells is remove by
rocytes are not inclu e in the total count but are note pipetting, push-we ge–type smears can subsequently be pre-
per 100 white bloo cells (WBCs). A total o at least 100 pare an staine or microscopic examination. T is tech-
leukocytes shoul be counte . Express the results as a per- nique is use ul in the per ormance o leukocyte i erential
centage o total leukocytes counte . counts on patients with extremely low total leukocyte counts
or in special testing proce ures.
Reporting Results
Re erence values, particularly the ban neutrophil percent- Specimen
age, may vary. Values or chil ren i er rom a ult re erence A reshly rawn specimen o ED A-anticoagulate whole
values. See insi e back cover or a ull iscussion o re erence bloo is nee e .
values.
Procedure
Procedure Notes 1. Centri uge the specimen o whole anticoagulate bloo
A well-ma e an well-staine smear is essential to the accu- or at least 5 minutes at 2,000 to 2,500 rpm.
racy o the i erential count. T e knowle ge an ability o 2. With a Pasteur pipette, remove most o the top plasma
the cell morphologist are critical to high-quality results. layer an iscar .
A minimum o 300 leukocytes must be within the accept- 3. T e inter ace layer along with a small amount o plasma
able working area, when the total leukocyte count is no less an a small volume o erythrocytes can then be remove
than 4 × 10 /L. T e neutrophils, monocytes, an lymphocytes using a Pasteur pipette.
9
shoul appear evenly istribute in the usable f el s o the 4. A rop o this suspension can be place on a microscope
f lm. Less than 2% o the leukocytes shoul be isrupte or sli e an a push-we ge smear prepare . Air- ry an stain.
noni entif able orms except in certain orms associate with Alternative Technique
pathological states. I a isrupte cell is clearly i entif able,
inclu e it in the i erential count. Classi y noni entif able A ref nement o the classic bu y coat technique has been
isrupte cells (smu ges or baskets) as “other,” an note them evelope or use with automate bloo smear equipment.
on the report i more than a ew are observe . In this technique, saline solution an 22% albumin are
Te bloo smear preparation techniques escribe in a e to the inter ace layer. T is enhancement pro uces bet-
Chapter 2 are commonly use in the laboratory or the prepa- ter cell separation on the peripheral smear an minimizes
ration o bloo smears. In certain circumstances, the prepa- the sprea ing arti act uring centri ugation. o a this
ration o a bu y coat peripheral bloo smear increases the enhancement to the basic technique:
accuracy o the leukocyte i erential count. 1. Procee rom step 3 above by trans erring the inter ace
layer to a isposable Wintrobe (ESR) tube. T is tube is
Preparation of Buffy Coat Smears place into a 16 × 100-mm test tube an centri uge or a
Principle minimum o 5 minutes at 2,000 rpm.
An anticoagulate specimen o whole bloo is centri uge to 2. A er centri ugation, the top plasma layer is remove with
physically separate the bloo into three layers: plasma, leu- a Pasteur pipette an iscar e . Remove approximately
kocytes an platelets, an erythrocytes. T e inter ace layer 0.03 mL o the inter ace layer an a small volume o eryth-
between the plasma an erythrocytes is re erre to as the rocytes an trans er to a 20-mL test tube or plastic vial.
(continued)