Page 40 - PRE-U STPM CHEMISTRY TERM 1
P. 40
Chemistry Term 1 STPM
(iii) The beryllium atom in BeCl is 4 electrons short (b) H + H + H – + – –
2
of octet. Hence, it will combine with
H
N
H
N
N
two NH molecules to achieve octet. H N H H H H H H N H H H N N H H N H N
3
5 (a) (b) H H H H H H H H H
x x x x
NH : Trigonal pyramidal (3 bond-pairs + 1 lone-pair)
sp 3 sp 3 3
+
O N NH : Tetrahedral (4 bond-pairs)
4
NH : Bent (2 bond-pairs + 2 lone-pairs)
–
x x x x x 2
• • •
H H H (c)
x • x • N N P P
H H
CI CI CI CI CI CI CI CI
(c) x π π •
sp • x CI CI CI CI
x x NCl : sp hybridisation
3
3
H • C x • N x PCl : sp hybridisation
sp 3 3
NCl has a larger bond angle. N is more electronegative
3
than P, the bonding electrons will be closer to N in
6 (a) sp 2 sp 2 NCl compared to P in PCl . Thurs, the bond-pair
3
3
repulsion in NCl is stronger and pushed the bonds
3
O further apart.
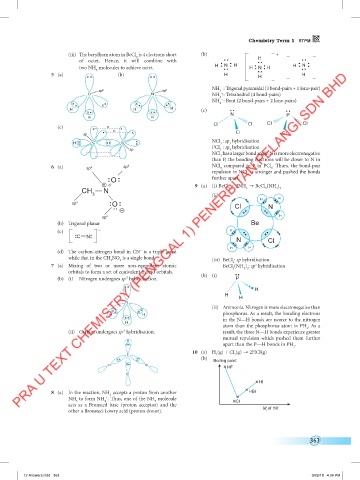
+ 9 (a) (i) BeCl + 2NH → BeCl (NH )
CH N 2 3 2 3 2
3 (ii)
H H
sp 3 O CI N
–
sp 3 H
(b) Trigonal planar Be
(c) – H
C N
N CI
H H
(d) The carbon-nitrogen bond in CN is a triple bond
–
while that in the CH NO is a single bond.
3 2 (iii) BeCl : sp hybridisation
2
7 (a) Mixing of two or more non-equivalent atomic BeCl (NH ) : sp hybridisation
3
3 2
2
orbitals to form a set of equivalent hybrid orbitals.
3
(b) (i) Nitrogen undergoes sp hybridisation. (b) (i) P
x x
H
H
H
N
(ii) Ammonia. Nitrogen is more electronegative than
x x •
H • H phosphorus. As a result, the bonding electrons
x • in the N—H bonds are nearer to the nitrogen
H
atom than the phosphorus atom in PH . As a
3
3
(ii) Carbon undergoes sp hybridisation. result, the three N—H bonds experience greater
mutual repulsion which pushed them further
H
x • apart than the P—H bonds in PH .
3
10 (a) H (g) + Cl (g) → 2HCl(g)
C 2 2
x • • x (b) Boiling point
x •
Cl Cl
HF
Cl
HI
8 (a) In the reaction, NH accepts a proton from another HBr
3
+
NH to form NH . Thus, one of the NH molecule HCI
3
4
3
acts as a Bronsted base (proton acceptor) and the M of HX
other a Bronsted-Lowry acid (proton donor). r
363
12 Answers.indd 363 3/26/18 4:06 PM