Page 47 - PRE-U STPM CHEMISTRY TERM 1
P. 47
Chemistry Term 1 STPM
STPM Practice 5 (b) (i)
Objective Questions In k
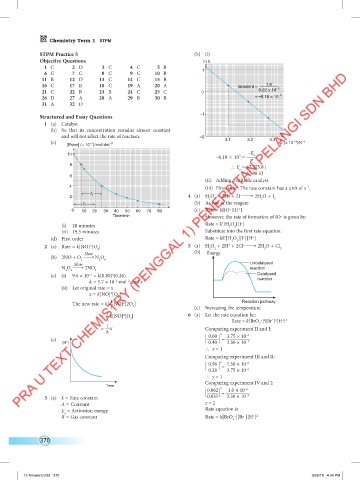
1 C 2 D 3 C 4 C 5 B 1
6 C 7 C 8 C 9 C 10 B
11 B 12 D 13 C 14 C 15 B
16 C 17 B 18 C 19 A 20 A Gradient = 1.8
21 C 22 B 23 B 24 C 25 C 0 0.22 × 10 –3
26 D 27 A 28 A 29 B 30 B = –8.18 × 10 –3
31 A 32 D
Structured and Essay Questions –1
1 (a) Catalyst
(b) So that its concentration remains almost constant
and will not affect the rate of reaction. –2
(c) 3.1 3.2 3.3 1 (× 10 )/K –1
–3
–1
[Ester] (× 10 )/mol dm –3 T
–E
10 3 a
–8.18 10 =
R
8
∴ E = 67 975.8 J
a
= 67.98 kJ
6
(ii) Adding a suitable catalyst
–1
4 (iii) First order. The rate constant has a unit of s .
t 2 + –
2 4 (a) H O + 2H + 2I → 2H O + I 2
2
2
2
(b) As one of the reagent
t 1
+
–
0 10 20 30 40 50 60 70 80 (c) Rate = k[IO ][H ]
–
Time/min However, the rate of formation of IO is given by:
Rate = k’[H O ][I ]
–
(i) 20 minutes 2 2
(ii) 19.5 minutes Substitute into the first rate equation:
+
–
(d) First order Rate = kk’[H O ][I ][H ]
2
2
+
–
2 (a) Rate = k[NO] [O ] 5 (a) H O + 2H + 2Cl → 2H O + Cl
2
2 2 2 2 2
Slow (b) Energy
(b) 2NO + O 9: N O
2 2 4
Slow Uncatalysed
N O 9: 2NO reaction
2 4 2
(c) (i) 9.6 × 10 = k(0.80) (0.26) Catalysed
–3
2
k = 5.7 × 10 mol dm s –1 reaction
–2
6
–2
(ii) Let original rate = x
x = k[NO] [O ]
2
2
1
The new rate = k[—NO] [2O ] Reaction pathway
2
4 2 (c) Increasing the temperature
1
2
= —k[NO] [O ] 6 (a) Let the rate equation be:
8 2 Rate = k[BrO ] [Br ] [H ]
– x
+ z
– y
3
1
= —x Comparing experiment II and I:
8 x –3
(c) 0.60 = 3.75 × 10 –3
[H ] + 0.40 2.50 × 10
∴ x = 1
Comparing experiment III and II:
y
0.56 = 7.50 × 10 –3
0.28 3.75 × 10 –3
∴ y = 1
Comparing experiment IV and I:
Time z –2
0.062 = 1.0 × 10 –3
3 (a) k = Rate constant 0.031 2.50 × 10
A = Constant z = 2
E = Activation energy Rate equation is:
a
–
–
+ 2
R = Gas constant Rate = k[BrO ][Br ][H ]
3
370
12 Answers.indd 370 3/26/18 4:06 PM