Page 46 - PRE-U STPM CHEMISTRY TERM 1
P. 46
Chemistry Term 1 STPM
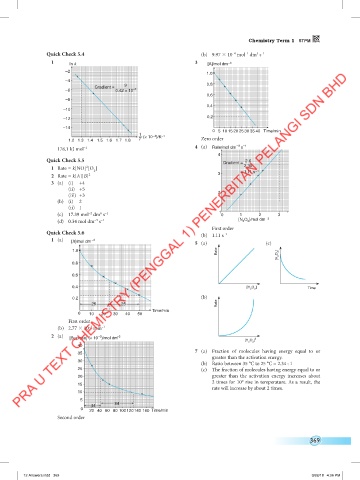
Quick Check 5.4 (b) 9.97 10 mol dm s
–1
–4
3 –1
1 In k 3 [A]/mol dm –3
–2
1.0
–4
9 0.8
Gradient =
–6 0.42 × 10 –3
0.6
–8
0.4
–10
0.2
–12
–14
0 5 10 15 20 25 30 35 40 Time/min
1 (× 10 )/K –1
–3
1.2 1.3 1.4 1.5 1.6 1.7 1.8 T Zero order
–3
–1
178.1 kJ mol 4 (a) Rate/mol dm s –1
4
Quick Check 5.5 2.6
Gradient =
2
1 Rate = k[NO] [O ] 2.35
2 = 1.11 s –1
2 Rate = k[A][B] 2 3
3 (a) (i) +4
(ii) +5 2
(iii) +3
(b) (i) 2
(ii) 1
–2
6 –1
(c) 17.39 mol dm s 1 0 1 2 3
(d) 0.34 mol dm s [N O ]/mol dm –3
–3 –1
5
2
First order
Quick Check 5.6 (b) 1.11 s –1
1 (a) [X]/mol dm –3 5 (a) (c)
Rate [H 2 O 2 ]
1.0
0.8
0.6
0.4 [H O ]
2 2 Time
(b)
0.2
Rate
25 25
Time/min
0 10 20 30 40 50
First order
–2
(b) 2.77 10 min –1
2 (a)
–2
[Sucrose] (× 10 )/mol dm –3 2
[H O ]
2 2
40
7 (a) Fraction of molecules having energy equal to or
35
greater than the activation energy.
30
(b) Ratio between 35 °C to 25 °C = 2.34 : 1
25 (c) The fraction of molecules having energy equal to or
20 greater than the activation energy increases about
2 times for 10° rise in temperature. As a result, the
15
rate will increase by about 2 times.
10
5
84
44
0 20 40 60 80 100120140 160 Time/min
Second order
369
12 Answers.indd 369 3/26/18 4:06 PM