Page 32 - Ranger SPM 2022 - Science
P. 32
Science SPM Chapter 6 Electrochemistry
6.1 Electrolytic Cell
Electrolysis
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
1. Electrolysis is the decomposition of a compound in the molten or aqueous
state into its constituent elements when electric current flows through it.
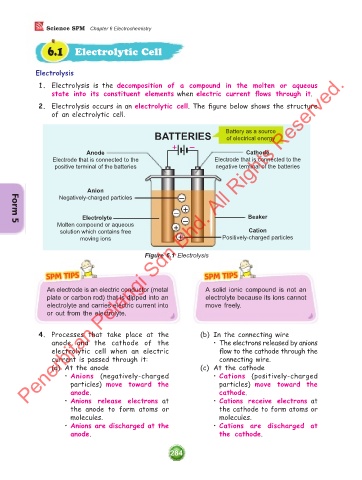
2. Electrolysis occurs in an electrolytic cell. The figure below shows the structure
of an electrolytic cell.
BATTERIES Battery as a source
of electrical energy
+ –
Anode Cathode
Electrode that is connected to the Electrode that is connected to the
positive terminal of the batteries negative terminal of the batteries
Anion
Negatively-charged particles –
– +
Electrolyte – Beaker
Molten compound or aqueous +
Form 5
solution which contains free Cation
moving ions + Positively-charged particles
Figure 6.1 Electrolysis
SPM TIPS SPM TIPS
An electrode is an electric conductor (metal A solid ionic compound is not an
plate or carbon rod) that is dipped into an electrolyte because its ions cannot
electrolyte and carries electric current into move freely.
or out from the electrolyte.
4. Processes that take place at the (b) In the connecting wire
anode and the cathode of the • The electrons released by anions
electrolytic cell when an electric flow to the cathode through the
current is passed through it: connecting wire.
(a) At the anode (c) At the cathode
• Anions (negatively-charged • Cations (positively-charged
particles) move toward the particles) move toward the
anode. cathode.
• Anions release electrons at • Cations receive electrons at
the anode to form atoms or the cathode to form atoms or
molecules. molecules.
• Anions are discharged at the • Cations are discharged at
anode. the cathode.
284
F5 Chapter 6.indd 284 3/21/22 3:59 PM