Page 33 - Ranger SPM 2022 - Science
P. 33
Science SPM Chapter 6 Electrochemistry
5. Electrolysis of an ionic compound in the molten state:
Example 1
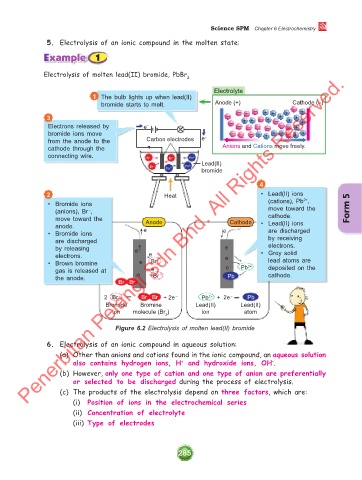
Electrolysis of molten lead(II) bromide, PbBr 2
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
Electrolyte
1 The bulb lights up when lead(II)
bromide starts to melt. Anode (+) Cathode (–)
–
+ +
3 – – – + – – + – + + + + +
+
Electrons released by e – – – – – – – – + +
bromide ions move – – – + + + + – + +
from the anode to the Carbon electrodes e –
cathode through the Anions and Cations move freely.
connecting wire. Br – Br – Pb 2+
Lead(II)
Br – Pb 2+
Pb 2+ bromide
4
2 Heat • Lead(II) ions
2+
• Bromide ions (cations), Pb ,
(anions), Br , move toward the Form 5
–
move toward the Anode Cathode cathode.
anode. • Lead(II) ions
• Bromide ions e e are discharged
are discharged by receiving
by releasing e e electrons.
electrons. e e • Grey solid
• Brown bromine e Br – 2+ lead atoms are
gas is released at e e Pb deposited on the
the anode. e Br – Pb cathode.
Br Br
2 Br – Br Br + 2e – Pb 2+ + 2e – Pb
Bromide Bromine Lead(II) Lead(II)
ion molecule (Br ) ion atom
2
Figure 6.2 Electrolysis of molten lead(II) bromide
6. Electrolysis of an ionic compound in aqueous solution:
(a) Other than anions and cations found in the ionic compound, an aqueous solution
also contains hydrogen ions, H and hydroxide ions, OH .
–
+
(b) However, only one type of cation and one type of anion are preferentially
or selected to be discharged during the process of electrolysis.
(c) The products of the electrolysis depend on three factors, which are:
(i) Position of ions in the electrochemical series
(ii) Concentration of electrolyte
(iii) Type of electrodes
285
F5 Chapter 6.indd 285 3/21/22 3:59 PM