Page 34 - Ranger SPM 2022 - Science
P. 34
Science SPM Chapter 6 Electrochemistry
(i) Position of ions in the electrochemical series
Example 2
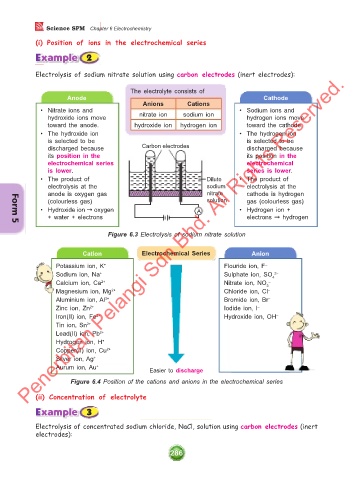
Electrolysis of sodium nitrate solution using carbon electrodes (inert electrodes):
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
The electrolyte consists of
Anode Cathode
Anions Cations
• Nitrate ions and • Sodium ions and
hydroxide ions move nitrate ion sodium ion hydrogen ions move
toward the anode. hydroxide ion hydrogen ion toward the cathode.
• The hydroxide ion • The hydrogen ion
is selected to be is selected to be
discharged because Carbon electrodes discharged because
its position in the its position in the
electrochemical series electrochemical
is lower. series is lower.
• The product of Dilute • The product of
electrolysis at the sodium electrolysis at the
anode is oxygen gas nitrate cathode is hydrogen
(colourless gas) solution gas (colourless gas)
• Hydroxide ion ➞ oxygen A • Hydrogen ion +
+ water + electrons electrons ➞ hydrogen
Form 5
Figure 6.3 Electrolysis of sodium nitrate solution
Cation Electrochemical Series Anion
Potassium ion, K + Flouride ion, F –
Sodium ion, Na + Sulphate ion, SO 4 2–
Calcium ion, Ca 2+ Nitrate ion, NO 3 –
Magnesium ion, Mg 2+ Chloride ion, Cl –
Aluminium ion, Al 3+ Bromide ion, Br –
Zinc ion, Zn 2+ Iodide ion, I –
Iron(II) ion, Fe 2+ Hydroxide ion, OH –
Tin ion, Sn 2+
Lead(II) ion, Pb 2+
Hydrogen ion, H +
Copper(II) ion, Cu 2+
Silver ion, Ag +
Aurum ion, Au +
Easier to discharge
Figure 6.4 Position of the cations and anions in the electrochemical series
(ii) Concentration of electrolyte
Example 3
Electrolysis of concentrated sodium chloride, NaCl, solution using carbon electrodes (inert
electrodes):
286
F5 Chapter 6.indd 286 3/21/22 3:59 PM