Page 13 - Ranger SPM 2022 Chemistry
P. 13
Chemistry SPM Chapter 2 Carbon Compound
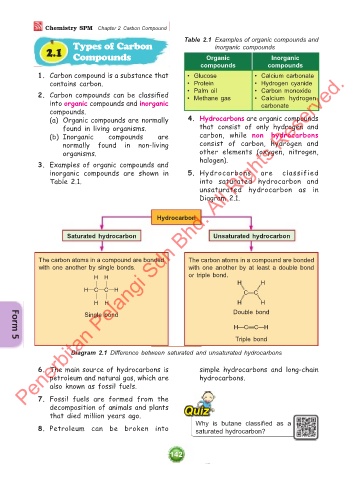
Table 2.1 Examples of organic compounds and
Types of Carbon
inorganic compounds
2.1 Compounds Organic Inorganic
compounds compounds
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
1. Carbon compound is a substance that • Glucose • Calcium carbonate
contains carbon. • Protein • Hydrogen cyanide
2. Carbon compounds can be classified • Palm oil • Carbon monoxide
• Calcium hydrogen
• Methane gas
into organic compounds and inorganic carbonate
compounds.
(a) Organic compounds are normally 4. Hydrocarbons are organic compounds
found in living organisms. that consist of only hydrogen and
(b) Inorganic compounds are carbon, while non hydrocarbons
normally found in non-living consist of carbon, hydrogen and
organisms. other elements (oxygen, nitrogen,
3. Examples of organic compounds and halogen).
inorganic compounds are shown in 5. Hydrocarbons are classified
Table 2.1. into saturated hydrocarbon and
unsaturated hydrocarbon as in
Diagram 2.1.
Hydrocarbon
Saturated hydrocarbon Unsaturated hydrocarbon
The carbon atoms in a compound are bonded The carbon atoms in a compound are bonded
with one another by single bonds. with one another by at least a double bond
H H or triple bond.
& & H H
H!C!C!H C"C
& &
H H H H
Single bond Double bond
H!C#C!H
Triple bond
Form 5
Diagram 2.1 Difference between saturated and unsaturated hydrocarbons
6. The main source of hydrocarbons is simple hydrocarbons and long-chain
petroleum and natural gas, which are hydrocarbons.
also known as fossil fuels.
7. Fossil fuels are formed from the
decomposition of animals and plants Quiz
Quiz
Quiz
that died million years ago. Quiz
Why is butane classified as a
8. Petroleum can be broken into saturated hydrocarbon?
142
02.5 RANGER SPM CHEMISTRY 2P.indd 142 29/03/2022 2:27 PM