Page 20 - Ranger SPM 2022 Chemistry
P. 20
Chemistry SPM Chapter 2 Carbon Compound
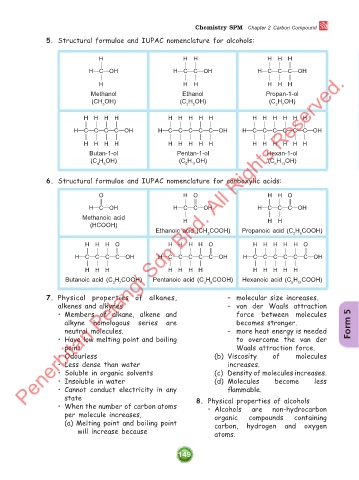
5. Structural formulae and IUPAC nomenclature for alcohols:
H H H H H H
& & & & & &
H!C!OH H!C!C!OH H!C!C!C!OH
Penerbitan Pelangi Sdn Bhd. All Rights Reserved.
& & & & & &
H H H H H H
Methanol Ethanol Propan-1-ol
(CH OH) (C H OH) (C H OH)
3 2 5 3 7
H H H H H H H H H H H H H H H
& & & & & & & & & & & & & & &
H!C!C!C!C!OH H!C!C!C!C!C!OH H!C!C!C!C!C!C!OH
& & & & & & & & & & & & & & &
H H H H H H H H H H H H H H H
Butan-1-ol Pentan-1-ol Hexan-1-ol
(C H OH) (C H OH) (C H OH)
4 9 5 11 6 13
6. Structural formulae and IUPAC nomenclature for carboxylic acids:
O H O H H O
' & ' & & '
H!C!OH H!C!C!OH H!C!C!C!OH
& & &
Methanoic acid H H H
(HCOOH)
Ethanoic acid (CH COOH) Propanoic acid (C H COOH)
3 2 5
H H H O H H H H O H H H H H O
& & & ' & & & & ' & & & & & '
H!C!C!C!C!OH H!C!C!C!C!C!OH H!C!C!C!C!C!C!OH
& & & & & & & & & & & &
H H H H H H H H H H H H
Butanoic acid (C H COOH) Pentanoic acid (C H COOH) Hexanoic acid (C H COOH)
3 7 4 9 5 11
7. Physical properties of alkanes, – molecular size increases.
alkenes and alkynes – van der Waals attraction
• Members of alkane, alkene and force between molecules
alkyne homologous series are becomes stronger. Form 5
neutral molecules. – more heat energy is needed
• Have low melting point and boiling to overcome the van der
point Waals attraction force.
• Odourless (b) Viscosity of molecules
• Less dense than water increases.
• Soluble in organic solvents (c) Density of molecules increases.
• Insoluble in water (d) Molecules become less
• Cannot conduct electricity in any flammable.
state 8. Physical properties of alcohols
• When the number of carbon atoms • Alcohols are non-hydrocarbon
per molecule increases, organic compounds containing
(a) Melting point and boiling point carbon, hydrogen and oxygen
will increase because atoms.
149
02.5 RANGER SPM CHEMISTRY 2P.indd 149 29/03/2022 2:28 PM