Page 15 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 15
Chemistry Terminologies
Chapter 7 : Ionic Equilibria
Chemistry Unit KMNS
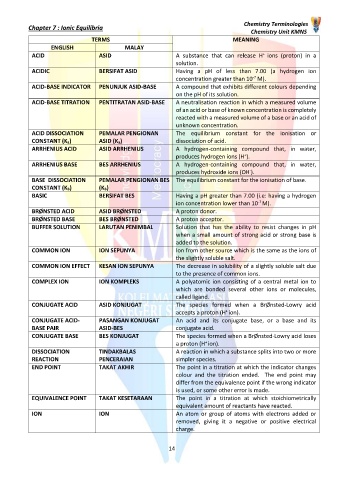
TERMS MEANING
ENGLISH MALAY
+
ACID ASID A substance that can release H ions (proton) in a
solution.
ACIDIC BERSIFAT ASID Having a pH of less than 7.00 (a hydrogen ion
-7
concentration greater than 10 M).
ACID-BASE INDICATOR PENUNJUK ASID-BASE A compound that exhibits different colours depending
on the pH of its solution.
ACID-BASE TITRATION PENTITRATAN ASID-BASE A neutralisation reaction in which a measured volume
of an acid or base of known concentration is completely
reacted with a measured volume of a base or an acid of
unknown concentration.
ACID DISSOCIATION PEMALAR PENGIONAN The equilibrium constant for the ionisation or
CONSTANT (Ka) ASID (Ka) dissociation of acid.
ARRHENIUS ACID ASID ARRHENIUS A hydrogen-containing compound that, in water,
+
produces hydrogen ions (H ).
ARRHENIUS BASE BES ARRHENIUS A hydrogen-containing compound that, in water,
-
produces hydroxide ions (OH ).
BASE DISSOCIATION PEMALAR PENGIONAN BES The equilibrium constant for the ionisation of base.
CONSTANT (KB) (KB)
BASIC BERSIFAT BES Having a pH greater than 7.00 (i.e: having a hydrogen
-7
ion concentration lower than 10 M).
BRØNSTED ACID ASID BRØNSTED A proton donor.
BRØNSTED BASE BES BRØNSTED A proton acceptor.
BUFFER SOLUTION LARUTAN PENIMBAL Solution that has the ability to resist changes in pH
when a small amount of strong acid or strong base is
added to the solution.
COMMON ION ION SEPUNYA Ion from other source which is the same as the ions of
the slightly soluble salt.
COMMON ION EFFECT KESAN ION SEPUNYA The decrease in solubility of a slightly soluble salt due
to the presence of common ions.
COMPLEX ION ION KOMPLEKS A polyatomic ion consisting of a central metal ion to
which are bonded several other ions or molecules,
called ligand.
CONJUGATE ACID ASID KONJUGAT The species formed when a BrØnsted-Lowry acid
+
accepts a proton (H ion).
CONJUGATE ACID- PASANGAN KONJUGAT An acid and its conjugate base, or a base and its
BASE PAIR ASID-BES conjugate acid.
CONJUGATE BASE BES KONJUGAT The species formed when a BrØnsted-Lowry acid loses
+
a proton (H ion).
DISSOCIATION TINDAKBALAS A reaction in which a substance splits into two or more
REACTION PENCERAIAN simpler species.
END POINT TAKAT AKHIR The point in a titration at which the indicator changes
colour and the titration ended. The end point may
differ from the equivalence point if the wrong indicator
is used, or some other error is made.
EQUIVALENCE POINT TAKAT KESETARAAN The point in a titration at which stoichiometrically
equivalent amount of reactants have reacted.
ION ION An atom or group of atoms with electrons added or
removed, giving it a negative or positive electrical
charge.
14