Page 10 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 10
Chemistry Terminologies
Chemistry Unit KMNS
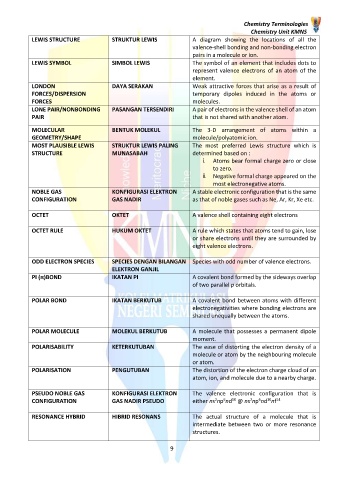
LEWIS STRUCTURE STRUKTUR LEWIS A diagram showing the locations of all the
valence-shell bonding and non-bonding electron
pairs in a molecule or ion.
LEWIS SYMBOL SIMBOL LEWIS The symbol of an element that includes dots to
represent valence electrons of an atom of the
element.
LONDON DAYA SERAKAN Weak attractive forces that arise as a result of
FORCES/DISPERSION temporary dipoles induced in the atoms or
FORCES molecules.
LONE PAIR/NONBONDING PASANGAN TERSENDIRI A pair of electrons in the valence shell of an atom
PAIR that is not shared with another atom.
MOLECULAR BENTUK MOLEKUL The 3-D arrangement of atoms within a
GEOMETRY/SHAPE molecule/polyatomic ion.
MOST PLAUSIBLE LEWIS STRUKTUR LEWIS PALING The most preferred Lewis structure which is
STRUCTURE MUNASABAH determined based on :
i. Atoms bear formal charge zero or close
to zero.
ii. Negative formal charge appeared on the
most electronegative atoms.
NOBLE GAS KONFIGURASI ELEKTRON A stable electronic configuration that is the same
CONFIGURATION GAS NADIR as that of noble gases such as Ne, Ar, Kr, Xe etc.
OCTET OKTET A valence shell containing eight electrons
OCTET RULE HUKUM OKTET A rule which states that atoms tend to gain, lose
or share electrons until they are surrounded by
eight valence electrons.
ODD ELECTRON SPECIES SPECIES DENGAN BILANGAN Species with odd number of valence electrons.
ELEKTRON GANJIL
PI (π)BOND IKATAN PI A covalent bond formed by the sideways overlap
of two parallel p orbitals.
POLAR BOND IKATAN BERKUTUB A covalent bond between atoms with different
electronegativities where bonding electrons are
shared unequally between the atoms.
POLAR MOLECULE MOLEKUL BERKUTUB A molecule that possesses a permanent dipole
moment.
POLARISABILITY KETERKUTUBAN The ease of distorting the electron density of a
molecule or atom by the neighbouring molecule
or atom.
POLARISATION PENGUTUBAN The distortion of the electron charge cloud of an
atom, ion, and molecule due to a nearby charge.
PSEUDO NOBLE GAS KONFIGURASI ELEKTRON The valence electronic configuration that is
10
2
6
10
6
2
CONFIGURATION GAS NADIR PSEUDO either ns np nd @ ns np nd nf 14
RESONANCE HYBRID HIBRID RESONANS The actual structure of a molecule that is
intermediate between two or more resonance
structures.
9