Page 14 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 14
Chemistry Terminologies
Chapter 6 : Chemical Equilibrium
Chemistry Unit KMNS
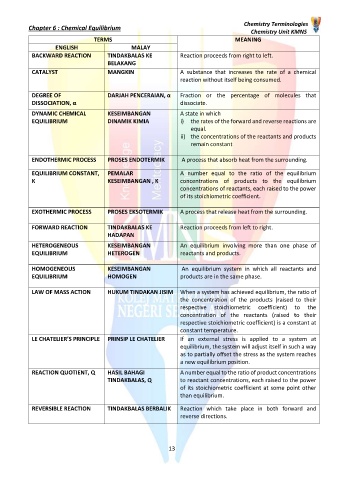
TERMS MEANING
ENGLISH MALAY
BACKWARD REACTION TINDAKBALAS KE Reaction proceeds from right to left.
BELAKANG
CATALYST MANGKIN A substance that increases the rate of a chemical
reaction without itself being consumed.
DEGREE OF DARJAH PENCERAIAN, α Fraction or the percentage of molecules that
DISSOCIATION, α dissociate.
DYNAMIC CHEMICAL KESEIMBANGAN A state in which
EQUILIBRIUM DINAMIK KIMIA i) the rates of the forward and reverse reactions are
equal.
ii) the concentrations of the reactants and products
remain constant
ENDOTHERMIC PROCESS PROSES ENDOTERMIK A process that absorb heat from the surrounding.
EQUILIBRIUM CONSTANT, PEMALAR A number equal to the ratio of the equilibrium
K KESEIMBANGAN , K concentrations of products to the equilibrium
concentrations of reactants, each raised to the power
of its stoichiometric coefficient.
EXOTHERMIC PROCESS PROSES EKSOTERMIK A process that release heat from the surrounding.
FORWARD REACTION TINDAKBALAS KE Reaction proceeds from left to right.
HADAPAN
HETEROGENEOUS KESEIMBANGAN An equilibrium involving more than one phase of
EQUILIBRIUM HETEROGEN reactants and products.
HOMOGENEOUS KESEIMBANGAN An equilibrium system in which all reactants and
EQUILIBRIUM HOMOGEN products are in the same phase.
LAW OF MASS ACTION HUKUM TINDAKAN JISIM When a system has achieved equilibrium, the ratio of
the concentration of the products (raised to their
respective stoichiometric coefficient) to the
concentration of the reactants (raised to their
respective stoichiometric coefficient) is a constant at
constant temperature.
LE CHATELIER’S PRINCIPLE PRINSIP LE CHATELIER If an external stress is applied to a system at
equilibrium, the system will adjust itself in such a way
as to partially offset the stress as the system reaches
a new equilibrium position.
REACTION QUOTIENT, Q HASIL BAHAGI A number equal to the ratio of product concentrations
TINDAKBALAS, Q to reactant concentrations, each raised to the power
of its stoichiometric coefficient at some point other
than equilibrium.
REVERSIBLE REACTION TINDAKBALAS BERBALIK Reaction which take place in both forward and
reverse directions.
13