Page 13 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 13
Chemistry Terminologies
Chemistry Unit KMNS
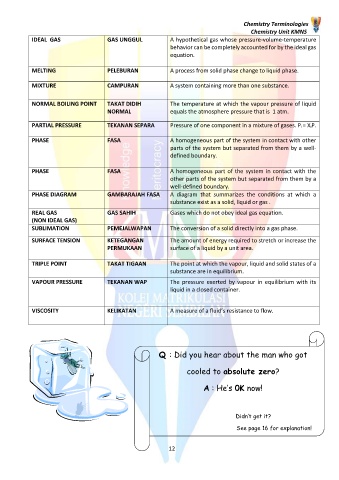
IDEAL GAS GAS UNGGUL A hypothetical gas whose pressure-volume-temperature
behavior can be completely accounted for by the ideal gas
equation.
MELTING PELEBURAN A process from solid phase change to liquid phase.
MIXTURE CAMPURAN A system containing more than one substance.
NORMAL BOILING POINT TAKAT DIDIH The temperature at which the vapour pressure of liquid
NORMAL equals the atmosphere pressure that is 1 atm.
PARTIAL PRESSURE TEKANAN SEPARA Pressure of one component in a mixture of gases. Pi = XiP.
PHASE FASA A homogeneous part of the system in contact with other
parts of the system but separated from them by a well-
defined boundary.
PHASE FASA A homogeneous part of the system in contact with the
other parts of the system but separated from them by a
well-defined boundary.
PHASE DIAGRAM GAMBARAJAH FASA A diagram that summarizes the conditions at which a
substance exist as a solid, liquid or gas .
REAL GAS GAS SAHIH Gases which do not obey ideal gas equation.
(NON IDEAL GAS)
SUBLIMATION PEMEJALWAPAN The conversion of a solid directly into a gas phase.
SURFACE TENSION KETEGANGAN The amount of energy required to stretch or increase the
PERMUKAAN surface of a liquid by a unit area.
TRIPLE POINT TAKAT TIGAAN The point at which the vapour, liquid and solid states of a
substance are in equilibrium.
VAPOUR PRESSURE TEKANAN WAP The pressure exerted by vapour in equilibrium with its
liquid in a closed container.
VISCOSITY KELIKATAN A measure of a fluid’s resistance to flow.
Q : Did you hear about the man who got
cooled to absolute zero?
A : He’s 0K now!
Didn’t get it?
See page 16 for explanation!
12