Page 9 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 9
Chemistry Terminologies
Chapter 4 : Chemical Bonding Chemistry Unit KMNS
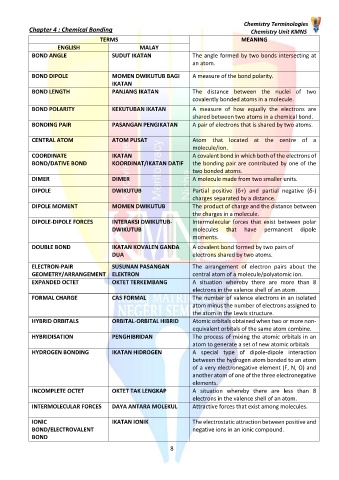
TERMS MEANING
ENGLISH MALAY
BOND ANGLE SUDUT IKATAN The angle formed by two bonds intersecting at
an atom.
BOND DIPOLE MOMEN DWIKUTUB BAGI A measure of the bond polarity.
IKATAN
BOND LENGTH PANJANG IKATAN The distance between the nuclei of two
covalently bonded atoms in a molecule.
BOND POLARITY KEKUTUBAN IKATAN A measure of how equally the electrons are
shared between two atoms in a chemical bond.
BONDING PAIR PASANGAN PENGIKATAN A pair of electrons that is shared by two atoms.
CENTRAL ATOM ATOM PUSAT Atom that located at the centre of a
molecule/ion.
COORDINATE IKATAN A covalent bond in which both of the electrons of
BOND/DATIVE BOND KOORDINAT/IKATAN DATIF the bonding pair are contributed by one of the
two bonded atoms.
DIMER DIMER A molecule made from two smaller units.
DIPOLE DWIKUTUB Partial positive (δ+) and partial negative (δ-)
charges separated by a distance.
DIPOLE MOMENT MOMEN DWIKUTUB The product of charge and the distance between
the charges in a molecule.
DIPOLE-DIPOLE FORCES INTERAKSI DWIKUTUB- Intermolecular forces that exist between polar
DWIKUTUB molecules that have permanent dipole
moments.
DOUBLE BOND IKATAN KOVALEN GANDA A covalent bond formed by two pairs of
DUA electrons shared by two atoms.
ELECTRON-PAIR SUSUNAN PASANGAN The arrangement of electron pairs about the
GEOMETRY/ARRANGEMENT ELEKTRON central atom of a molecule/polyatomic ion.
EXPANDED OCTET OKTET TERKEMBANG A situation whereby there are more than 8
electrons in the valence shell of an atom.
FORMAL CHARGE CAS FORMAL The number of valence electrons in an isolated
atom minus the number of electrons assigned to
the atom in the Lewis structure.
HYBRID ORBITALS ORBITAL-ORBITAL HIBRID Atomic orbitals obtained when two or more non-
equivalent orbitals of the same atom combine.
HYBRIDISATION PENGHIBRIDAN The process of mixing the atomic orbitals in an
atom to generate a set of new atomic orbitals
HYDROGEN BONDING IKATAN HIDROGEN A special type of dipole-dipole interaction
between the hydrogen atom bonded to an atom
of a very electronegative element (F, N, O) and
another atom of one of the three electronegative
elements.
INCOMPLETE OCTET OKTET TAK LENGKAP A situation whereby there are less than 8
electrons in the valence shell of an atom.
INTERMOLECULAR FORCES DAYA ANTARA MOLEKUL Attractive forces that exist among molecules.
IONIC IKATAN IONIK The electrostatic attraction between positive and
BOND/ELECTROVALENT negative ions in an ionic compound.
BOND
8