Page 8 - Chemistry Terminologies SK015 & DK014_Chemistry Unit, KMNS
P. 8
Chemistry Terminologies
Chemistry Unit KMNS
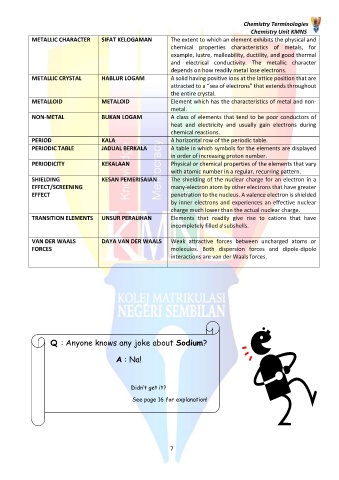
METALLIC CHARACTER SIFAT KELOGAMAN The extent to which an element exhibits the physical and
chemical properties characteristics of metals, for
example, lustre, malleability, ductility, and good thermal
and electrical conductivity. The metallic character
depends on how readily metal lose electrons.
METALLIC CRYSTAL HABLUR LOGAM A solid having positive ions at the lattice position that are
attracted to a “sea of electrons” that extends throughout
the entire crystal.
METALLOID METALOID Element which has the characteristics of metal and non-
metal.
NON-METAL BUKAN LOGAM A class of elements that tend to be poor conductors of
heat and electricity and usually gain electrons during
chemical reactions.
PERIOD KALA A horizontal row of the periodic table.
PERIODIC TABLE JADUAL BERKALA A table in which symbols for the elements are displayed
in order of increasing proton number.
PERIODICITY KEKALAAN Physical or chemical properties of the elements that vary
with atomic number in a regular, recurring pattern.
SHIELDING KESAN PEMERISAIAN The shielding of the nuclear charge for an electron in a
EFFECT/SCREENING many-electron atom by other electrons that have greater
EFFECT penetration to the nucleus. A valence electron is shielded
by inner electrons and experiences an effective nuclear
charge much lower than the actual nuclear charge.
TRANSITION ELEMENTS UNSUR PERALIHAN Elements that readily give rise to cations that have
incompletely filled d subshells.
VAN DER WAALS DAYA VAN DER WAALS Weak attractive forces between uncharged atoms or
FORCES molecules. Both dispersion forces and dipole-dipole
interactions are van der Waals forces.
Q : Anyone knows any joke about Sodium?
A : Na!
Didn’t get it?
See page 16 for explanation!
7