Page 28 - Drug Discovery and Development: Prospects and Challenges
P. 28
Figure 14: Mature adipocyte stained with oil red O
The effects of several phytochemical compounds have been studied on 3T3-L1 adipocytes,
such as cinnamtannin B1, which is a type of proanthocyanidin from Cinnamomum zeylanicum (Figure
15). Cinnamtannin B1 showed an improved glucose uptake in 3T3-L1 preadipocytes. In our study, we
have concluded that the sweet agent cinnamtannin B1 in cinnamon mimics insulin by attaching to the
β-subunit of the receptor in the cell membrane, followed by autophosphorylating the tyrosine residues
of the β-subunit. Subsequently, the phosphoinositide 3-kinases (PI3K), glucose transporter 4 (GLUT4)
translocation, and glucose absorption were activated. Based on the response of the cinnamtannin B1,
it was concluded that the phosphorylation of the insulin receptor (IR )β-subunit is an important
molecular target for the treatment of diabetes (Taher et al., 2004, 2006, 2007).
Fascinatingly, our finding had attracted the attention of the national newspaper at that time with
an article “Sweet hope for diabetics” (Abdullah, 2006). The study was supported by a clinical trial on
aqueous cinnamon extract in which 120 mg (low dose) and 360 mg (high dose) mg was given to
patients with type 2 diabetes daily for three months. The results showed that the HbA1c and fasting
plasma glucose levels significantly decreased (reduction of 1.62 mmol/L vs. 0.22 mmol/L in the
placebo group) (Lu et al., 2012). Based on a systematic review and meta-analysis studies involving
1025 participants, the supplementation of cinnamon was found to decrease serum triglycerides, total
18 / Drug Discovery and Development: Prospects and Challenges
cholesterol, and low-density lipoprotein cholesterol (Jamali et al., 2020).
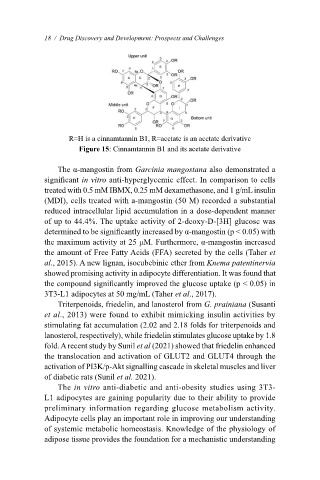
R=H is a cinnamtannin B1, R=acetate is an acetate derivative
Figure 15: Cinnamtannin B1 and its acetate derivative
22
The α-mangostin from Garcinia mangostana also demonstrated a
significant in vitro anti-hyperglycemic effect. In comparison to cells
treated with 0.5 mM IBMX, 0.25 mM dexamethasone, and 1 g/mL insulin
(MDI), cells treated with a-mangostin (50 M) recorded a substantial
reduced intracellular lipid accumulation in a dose-dependent manner
of up to 44.4%. The uptake activity of 2-deoxy-D-[3H] glucose was
determined to be significantly increased by α-mangostin (p < 0.05) with
the maximum activity at 25 μM. Furthermore, α-mangostin increased
the amount of Free Fatty Acids (FFA) secreted by the cells (Taher et
al., 2015). A new lignan, isocubebinic ether from Knema patentinervia
showed promising activity in adipocyte differentiation. It was found that
the compound significantly improved the glucose uptake (p < 0.05) in
3T3-L1 adipocytes at 50 mg/mL (Taher et al., 2017).
Triterpenoids, friedelin, and lanosterol from G. prainiana (Susanti
et al., 2013) were found to exhibit mimicking insulin activities by
stimulating fat accumulation (2.02 and 2.18 folds for triterpenoids and
lanosterol, respectively), while friedelin stimulates glucose uptake by 1.8
fold. A recent study by Sunil et al (2021) showed that friedelin enhanced
the translocation and activation of GLUT2 and GLUT4 through the
activation of PI3K/p-Akt signalling cascade in skeletal muscles and liver
of diabetic rats (Sunil et al. 2021).
The in vitro anti-diabetic and anti-obesity studies using 3T3-
L1 adipocytes are gaining popularity due to their ability to provide
preliminary information regarding glucose metabolism activity.
Adipocyte cells play an important role in improving our understanding
of systemic metabolic homeostasis. Knowledge of the physiology of
adipose tissue provides the foundation for a mechanistic understanding