Page 161 - text book form physics kssm 2020
P. 161
Chapter 4
Heat
3
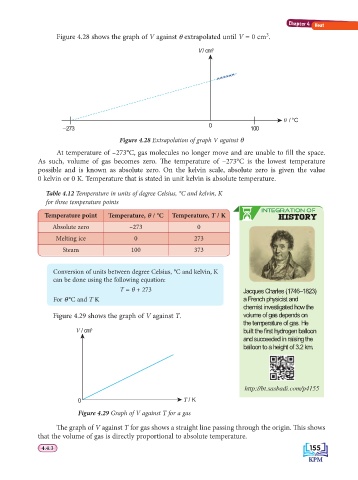
Figure 4.28 shows the graph of V against θ extrapolated until V = 0 cm .
V / cm 3
e / °C
0
–273 100
Figure 4.28 Extrapolation of graph V against θ
At temperature of –273°C, gas molecules no longer move and are unable to fi ll the space.
As such, volume of gas becomes zero. Th e temperature of –273°C is the lowest temperature
possible and is known as absolute zero. On the kelvin scale, absolute zero is given the value
0 kelvin or 0 K. Temperature that is stated in unit kelvin is absolute temperature.
Table 4.12 Temperature in units of degree Celsius, °C and kelvin, K
for three temperature points
INTEGRATION OF
Temperature point Temperature, θ / °C Temperature, T / K HISTORY
Absolute zero –273 0
Melting ice 0 273
Steam 100 373
Conversion of units between degree Celsius, °C and kelvin, K
can be done using the following equation:
T = θ + 273 Jacques Charles (1746–1823)
For θ °C and T K a French physicist and
chemist investigated how the
Figure 4.29 shows the graph of V against T. volume of gas depends on
the temperature of gas. He
V / cm 3 built the fi rst hydrogen balloon
and succeeded in raising the
balloon to a height of 3.2 km.
http://bt.sasbadi.com/p4155
0 T / K
Figure 4.29 Graph of V against T for a gas
Th e graph of V against T for gas shows a straight line passing through the origin. Th is shows
that the volume of gas is directly proportional to absolute temperature.
155
4.4.3 155
4.4.3