Page 32 - (DK) Ocean - The Definitive Visual Guide
P. 32
30 OCEAN WATER
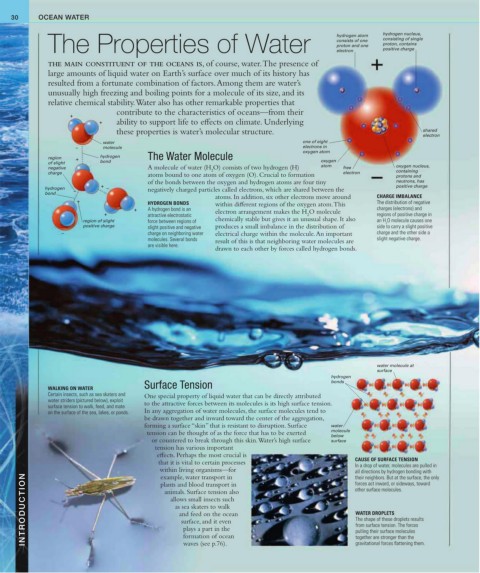
hydrogen nucleus,
The Properties of Water consists of one consisting of single
hydrogen atom
proton, contains
proton and one
positive charge
electron
THE MAIN CONSTITUENT OF THE OCEANS IS, of course, water. The presence of
large amounts of liquid water on Earth’s surface over much of its history has
resulted from a fortunate combination of factors. Among them are water’s
unusually high freezing and boiling points for a molecule of its size, and its
relative chemical stability. Water also has other remarkable properties that
contribute to the characteristics of oceans—from their
+
+ ability to support life to effects on climate. Underlying
these properties is water’s molecular structure. shared
electron
water one of eight
– molecule electrons in
oxygen atom
region + hydrogen The Water Molecule oxygen
of slight bond
negative A molecule of water (H O) consists of two hydrogen (H) atom free oxygen nucleus,
2
charge electron containing
atoms bound to one atom of oxygen (O). Crucial to formation protons and
of the bonds between the oxygen and hydrogen atoms are four tiny neutrons, has
+ positive charge
hydrogen – negatively charged particles called electrons, which are shared between the
bond –
atoms. In addition, six other electrons move around CHARGE IMBALANCE
+ HYDROGEN BONDS within different regions of the oxygen atom. This The distribution of negative
+ A hydrogen bond is an charges (electrons) and
+ attractive electrostatic electron arrangement makes the H O molecule regions of positive charge in
2
region of slight force between regions of chemically stable but gives it an unusual shape. It also an H O molecule causes one
2
positive charge slight positive and negative produces a small imbalance in the distribution of side to carry a slight positive
– charge on neighboring water electrical charge within the molecule. An important charge and the other side a
molecules. Several bonds result of this is that neighboring water molecules are slight negative charge.
are visible here.
drawn to each other by forces called hydrogen bonds.
water molecule at
surface
hydrogen
Surface Tension bonds
WALKING ON WATER
Certain insects, such as sea skaters and One special property of liquid water that can be directly attributed
water striders (pictured below), exploit
surface tension to walk, feed, and mate to the attractive forces between its molecules is its high surface tension.
on the surface of the sea, lakes, or ponds. In any aggregation of water molecules, the surface molecules tend to
be drawn together and inward toward the center of the aggregation,
forming a surface “skin” that is resistant to disruption. Surface water
tension can be thought of as the force that has to be exerted molecule
below
or countered to break through this skin. Water’s high surface surface
tension has various important
effects. Perhaps the most crucial is
CAUSE OF SURFACE TENSION
that it is vital to certain processes In a drop of water, molecules are pulled in
within living organisms—for all directions by hydrogen bonding with
INTRODUCTION animals. Surface tension also WATER DROPLETS
example, water transport in
their neighbors. But at the surface, the only
forces act inward, or sideways, toward
plants and blood transport in
other surface molecules.
allows small insects such
as sea skaters to walk
and feed on the ocean
The shape of these droplets results
surface, and it even
from surface tension. The forces
plays a part in the
pulling their surface molecules
formation of ocean
together are stronger than the
waves (see p.76).
gravitational forces flattening them.