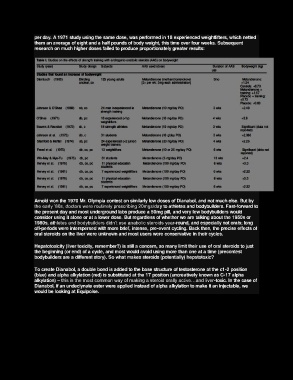
Page 15 - 02ed2018
P. 15
per day. A 1971 study using the same dose, was performed in 18 experienced weightlifters, which netted
them an average of eight and a half pounds of body weight, this time over four weeks. Subsequent
research on much higher doses failed to produce proportionately greater results:
Arnold won the 1970 Mr. Olympia contest on similarly low doses of Dianabol, and not much else. But by
the early ’80s, doctors were routinely prescribing 20mgs/day to athletes and bodybuilders. Fast-forward to
the present day and most underground labs produce a 50mg pill, and very few bodybuilders would
consider using it alone or at a lower dose. But regardless of whether we are talking about the 1950s or
1980s, athletes and bodybuilders didn’t use anabolic steroids year-round, and especially not orals; long
off-periods were interspersed with more brief, intense, pre-event cycling. Back then, the precise effects of
oral steroids on the liver were unknown and most users were conservative in their cycles.
Hepatotoxicity (liver toxicity, remember?) is still a concern, so many limit their use of oral steroids to just
the beginning (or end) of a cycle, and most would avoid using more than one at a time (precontest
bodybuilders are a different story). So what makes steroids (potentially) hepatotoxic?
To create Dianabol, a double bond is added to the base structure of testosterone at the c1-2 position
(blue) and alpha alkylation (red) is substituted at the 17 position (uncreatively known as C-17 alpha
alkylation) – this is the most common way of making a steroid orally active…and liver-toxic. In the case of
Dianabol, if an undeclynate ester were applied instead of alpha alkylation to make it an injectable, we
would be looking at Equipoise.