Page 101 - Nilam_Publication_module_Chemistry_Form.pdf
P. 101
Chemistry Form 4 • MODULE
EXERCISE / LATIHAN
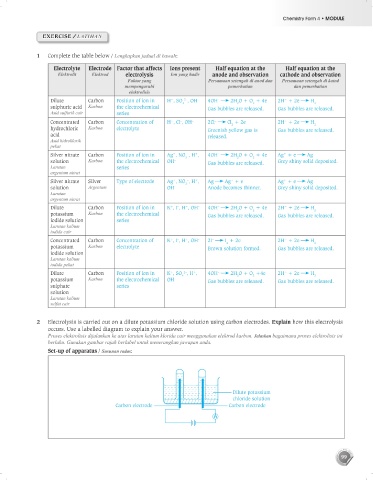
1 Complete the table below / Lengkapkan jadual di bawah:
Electrolyte Electrode Factor that affects Ions present Half equation at the Half equation at the
Elektrolit Elektrod electrolysis Ion yang hadir anode and observation cathode and observation
Faktor yang Persamaan setengah di anod dan Persamaan setengah di katod
mempengaruhi pemerhatian dan pemerhatian
elektrolisis
Dilute Carbon Position of ion in H , SO , OH – 4OH 2H O + O + 4e 2H + 2e H 2
–
+
+
2–
4
2
2
sulphuric acid Karbon the electrochemical Gas bubbles are released. Gas bubbles are released.
Asid sulfurik cair series
–
–
+
+
Concentrated Carbon Concentration of H , Cl , OH – 2Cl Cl + 2e 2H + 2e H 2
2
hydrochloric Karbon electrolyte Greenish yellow gas is Gas bubbles are released.
acid released.
Asid hidroklorik
pekat
Silver nitrate Carbon Position of ion in Ag , NO , H , 4OH 2H O + O + 4e Ag + e Ag
–
+
–
+
+
2
2
3
solution Karbon the electrochemical OH – Gas bubbles are released. Grey shiny solid deposited.
Larutan series
argentum nitrat
Silver nitrate Silver Type of electrode Ag , NO , H , Ag Ag + e Ag + e Ag
+
+
+
–
+
3
solution Argentum OH – Anode becomes thinner. Grey shiny solid deposited.
Larutan
argentum nitrat
–
–
Dilute Carbon Position of ion in K , I , H , OH – 4OH 2H O + O + 4e 2H + 2e H 2
+
+
+
2
2
potassium Karbon the electrochemical Gas bubbles are released. Gas bubbles are released.
iodide solution series
Larutan kalium
iodida cair
+
Concentrated Carbon Concentration of K , I , H , OH – 2I I + 2e 2H + 2e H 2
+
+
–
–
2
potassium Karbon electrolyte Brown solution formed. Gas bubbles are released.
iodide solution
Larutan kalium
iodida pekat
Dilute Carbon Position of ion in K , SO , H , 4OH 2H O + O +4e 2H + 2e H 2
+
–
+
2–
+
2
4
2
potassium Karbon the electrochemical OH – Gas bubbles are released. Gas bubbles are released.
sulphate series
solution
Larutan kalium
sulfat cair
2 Electrolysis is carried out on a dilute potassium chloride solution using carbon electrodes. Explain how this electrolysis
occurs. Use a labelled diagram to explain your answer.
Proses elektrolisis dijalankan ke atas larutan kalium klorida cair menggunakan elektrod karbon. Jelaskan bagaimana proses elektrolisis ini
berlaku. Gunakan gambar rajah berlabel untuk menerangkan jawapan anda.
Set-up of apparatus / Susunan radas:
Dilute potassium
chloride solution
Carbon electrode Carbon electrode
99
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 99 12/9/2011 5:56:30 PM