Page 100 - Nilam_Publication_module_Chemistry_Form.pdf
P. 100
MODULE • Chemistry Form 4
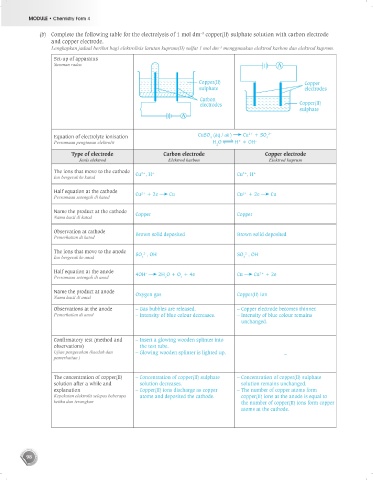
(b) Complete the following table for the electrolysis of 1 mol dm copper(II) sulphate solution with carbon electrode
–3
and copper electrode.
Lengkapkan jadual berikut bagi elektrolisis larutan kuprum(II) sulfat 1 mol dm menggunakan elektrod karbon dan elektrod kuprum.
–3
Set-up of apparatus
Susunan radas
Copper(II) Copper
sulphate electrodes
Carbon
electrodes Copper(II)
sulphate
2+
Equation of electrolyte ionisation CuSO (aq / ak ) Cu + SO 4 2–
4
+
Persamaan pengionan elektrolit H O H + OH –
2
Type of electrode Carbon electrode Copper electrode
Jenis elektrod Elektrod karbon Elektrod kuprum
The ions that move to the cathode Cu , H + Cu , H +
2+
2+
Ion bergerak ke katod
Half equation at the cathode Cu + 2e Cu Cu + 2e Cu
2+
2+
Persamaan setengah di katod
Name the product at the cathode Copper Copper
Nama hasil di katod
Observation at cathode Brown solid deposited Brown solid deposited
Pemerhatian di katod
The ions that move to the anode SO , OH – SO , OH –
2–
2–
Ion bergerak ke anod 4 4
Half equation at the anode 4OH 2H O + O + 4e Cu Cu + 2e
–
2+
Persamaan setengah di anod 2 2
Name the product at anode Oxygen gas Copper(II) ion
Nama hasil di anod
Observations at the anode – Gas bubbles are released. – Copper electrode becomes thinner.
Pemerhatian di anod – Intensity of blue colour decreases. – Intensity of blue colour remains
unchanged.
Confirmatory test (method and – Insert a glowing wooden splinter into
observations) the test tube.
Ujian pengesahan (kaedah dan – Glowing wooden splinter is lighted up. –
pemerhatian )
The concentration of copper(II) – Concentration of copper(II) sulphate – Concentration of copper(II) sulphate
solution after a while and solution decreases. solution remains unchanged.
explanation – Copper(II) ions discharge as copper – The number of copper atoms form
Kepekatan elektrolit selepas beberapa atoms and deposited the cathode. copper(II) ions at the anode is equal to
ketika dan terangkan the number of copper(II) ions form copper
atoms at the cathode.
98
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 98 12/9/2011 5:56:29 PM