Page 97 - Nilam_Publication_module_Chemistry_Form.pdf
P. 97
Chemistry Form 4 • MODULE
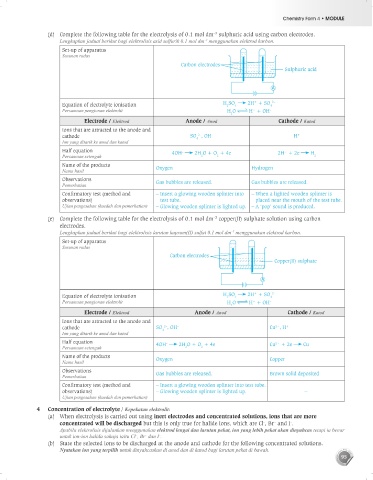
(d) Complete the following table for the electrolysis of 0.1 mol dm sulphuric acid using carbon electrodes.
–3
–3
Lengkapkan jadual berikut bagi elektrolisis asid sulfurik 0.1 mol dm menggunakan elektrod karbon.
Set-up of apparatus
Susunan radas
Carbon electrodes
Sulphuric acid
+
Equation of electrolyte ionisation H SO 2H + SO 4 2–
4
2
Persamaan pengionan elektrolit H O H + OH –
+
2
Electrode / Elektrod Anode / Anod Cathode / Katod
Ions that are attracted to the anode and
2–
cathode SO , OH – H +
4
Ion yang ditarik ke anod dan katod
Half equation 4OH 2H O + O + 4e 2H + 2e H
+
–
Persamaan setengah 2 2 2
Name of the products Oxygen Hydrogen
Nama hasil
Observations Gas bubbles are released. Gas bubbles are released.
Pemerhatian
Confirmatory test (method and – Insert a glowing wooden splinter into – When a lighted wooden splinter is
observations) test tube. placed near the mouth of the test tube.
Ujian pengesahan (kaedah dan pemerhatian) – Glowing wooden splinter is lighted up. – A ‘pop’ sound is produced.
(e) Complete the following table for the electrolysis of 0.1 mol dm copper(II) sulphate solution using carbon
–3
electrodes.
Lengkapkan jadual berikut bagi elektrolisis larutan kuprum(II) sulfat 0.1 mol dm menggunakan elektrod karbon.
–3
Set-up of apparatus
Susunan radas
Carbon electrodes
Copper(II) sulphate
+
Equation of electrolyte ionisation H SO 2H + SO 4 2–
2
4
Persamaan pengionan elektrolit H O H + OH –
+
2
Electrode / Elektrod Anode / Anod Cathode / Katod
Ions that are attracted to the anode and
cathode SO , OH – Cu , H +
2+
2–
4
Ion yang ditarik ke anod dan katod
Half equation 4OH 2H O + O + 4e Cu + 2e Cu
2+
–
Persamaan setengah 2 2
Name of the products Oxygen Copper
Nama hasil
Observations Gas bubbles are released. Brown solid deposited
Pemerhatian
Confirmatory test (method and – Insert a glowing wooden splinter into test tube.
observations) – Glowing wooden splinter is lighted up. –
Ujian pengesahan (kaedah dan pemerhatian)
4 Concentration of electrolyte / Kepekatan elektrolit:
(a) When electrolysis is carried out using inert electrodes and concentrated solutions, ions that are more
concentrated will be discharged but this is only true for halide ions, which are Cl , Br and I .
–
–
–
Apabila elektrolisis dijalankan menggunakan elektrod lengai dan larutan pekat, ion yang lebih pekat akan dinyahcas tetapi ia benar
–
untuk ion-ion halida sahaja iaitu Cl , Br dan I . –
–
(b) State the selected ions to be discharged at the anode and cathode for the following concentrated solutions.
Nyatakan ion yang terpilih untuk dinyahcaskan di anod dan di katod bagi larutan pekat di bawah.
95
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 95 12/9/2011 5:56:29 PM