Page 99 - Nilam_Publication_module_Chemistry_Form.pdf
P. 99
Chemistry Form 4 • MODULE
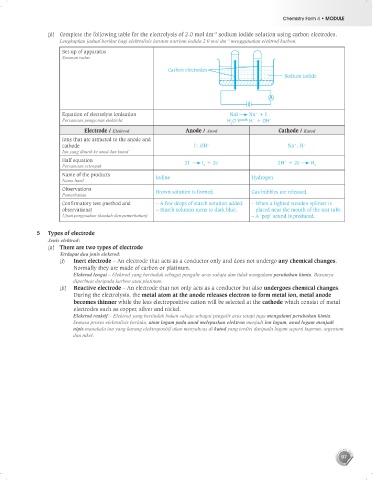
(d) Complete the following table for the electrolysis of 2.0 mol dm sodium iodide solution using carbon electrodes.
–3
Lengkapkan jadual berikut bagi elektrolisis larutan natrium iodida 2.0 mol dm menggunakan elektrod karbon.
–3
Set-up of apparatus
Susunan radas
Carbon electrodes
Sodium iodide
Equation of electrolyte ionisation NaI Na + I –
+
Persamaan pengionan elektrolit H O H + OH –
+
2
Electrode / Elektrod Anode / Anod Cathode / Katod
Ions that are attracted to the anode and
–
cathode I , OH – Na , H +
+
Ion yang ditarik ke anod dan katod
Half equation 2I I + 2e 2H + 2e H
–
+
Persamaan setengah 2 2
Name of the products Iodine Hydrogen
Nama hasil
Observations Brown solution is formed. Gas bubbles are released.
Pemerhatian
Confirmatory test (method and – A few drops of starch solution added. – When a lighted wooden splinter is
observations) – Starch solution turns to dark blue. placed near the mouth of the test tube.
Ujian pengesahan (kaedah dan pemerhatian) – A ‘pop’ sound is produced.
5 Types of electrode
Jenis elektrod:
(a) There are two types of electrode
Terdapat dua jenis elektrod:
(i) Inert electrode – An electrode that acts as a conductor only and does not undergo any chemical changes.
Normally they are made of carbon or platinum.
Elektrod lengai – Elektrod yang bertindak sebagai pengalir arus sahaja dan tidak mengalami perubahan kimia. Biasanya
diperbuat daripada karbon atau platinum.
(ii) Reactive electrode – An electrode that not only acts as a conductor but also undergoes chemical changes.
During the electrolysis, the metal atom at the anode releases electron to form metal ion, metal anode
becomes thinner while the less electropositive cation will be selected at the cathode which consist of metal
electrodes such as copper, silver and nickel.
Elektrod reaktif – Elektrod yang bertindak bukan sahaja sebagai pengalir arus tetapi juga mengalami perubahan kimia.
Semasa proses elektrolisis berlaku, atom logam pada anod melepaskan elektron menjadi ion logam, anod logam menjadi
nipis manakala ion yang kurang elektropositif akan menyahcas di katod yang terdiri daripada logam seperti kuprum, argentum
dan nikel.
97
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 97 12/9/2011 5:56:29 PM