Page 96 - Nilam_Publication_module_Chemistry_Form.pdf
P. 96
MODULE • Chemistry Form 4
(b) Choose the ion to be discharged from the following pairs of ions. State the electrode where it occurs and write
the half equation for the discharge of ion:
Pilih ion yang akan dinyahcas dari pasangan ion berikut, nyatakan di elektrod mana ia berlaku dan tulis persamaan setengah untuk
nyahcas ion:
–
(i) Hydroxide & sulphate ions : Half equation: 4OH 2H O + O + 4e at the anode .
2
2
–
4OH 2H O + O + 4e anod
Ion hidroksida & ion sulfat : Persamaan setengah: 2 2 di .
–
(ii) Hydroxide & nitrate ions : Half equation: 4OH 2H O + O + 4e at the anode .
2
2
4OH 2H O + O + 4e anod
–
Ion hidroksida & ion nitrat : Persamaan setengah: 2 2 di .
2+
(iii) Hydrogen & copper(II) ions : Half equation: Cu + 2e Cu at the cathode .
2+
Cu + 2e Cu katod
Ion hidrogen & ion kuprum(II) : Persamaan setengah: di .
+
(iv) Hydrogen & potassium ions : Half equation: 2H + 2e H 2 at the cathode .
2H + 2e H katod
+
Ion hidrogen & ion kalium : Persamaan setengah: 2 di .
+
(v) Hydrogen & silver ions : Half equation: Ag + e Ag at the cathode .
+
Ag + e Ag katod
Ion hidrogen & ion argentum : Persamaan setengah: di .
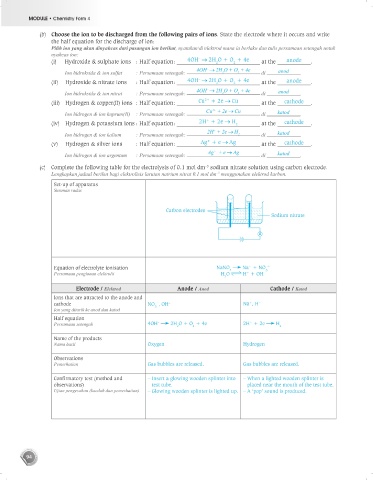
–3
(c) Complete the following table for the electrolysis of 0.1 mol dm sodium nitrate solution using carbon electrode.
Lengkapkan jadual berikut bagi elektrolisis larutan natrium nitrat 0.1 mol dm menggunakan elektrod karbon.
–3
Set-up of apparatus
Susunan radas
Carbon electrodes
Sodium nitrate
Equation of electrolyte ionisation NaNO Na + NO 3 –
+
3
Persamaan pengionan elektrolit H O H + OH –
+
2
Electrode / Elektrod Anode / Anod Cathode / Katod
Ions that are attracted to the anode and
–
+
cathode NO , OH – Na , H +
Ion yang ditarik ke anod dan katod 3
Half equation
–
+
Persamaan setengah 4OH 2H O + O + 4e 2H + 2e H 2
2
2
Name of the products
Nama hasil Oxygen Hydrogen
Observations
Pemerhatian Gas bubbles are released. Gas bubbles are released.
Confirmatory test (method and – Insert a glowing wooden splinter into – When a lighted wooden splinter is
observations) test tube. placed near the mouth of the test tube.
Ujian pengesahan (kaedah dan pemerhatian) – Glowing wooden splinter is lighted up. – A ‘pop’ sound is produced.
94
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 94 12/9/2011 5:56:29 PM