Page 54 - Nilam_Publication_module_Chemistry_Form.pdf
P. 54
MODULE • Chemistry Form 4
#
(e) Group 18: Noble gases / Kumpulan 18: Gas adi #
#The important groups that will be studied with respect to chemical and physical properties.
# Kumpulan penting yang akan dipelajari dari segi sifat fizik dan sifat kimia.
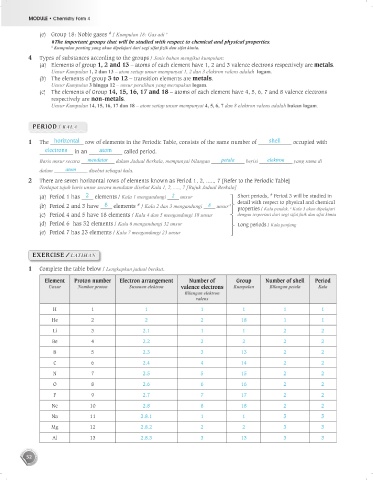
4 Types of substances according to the groups / Jenis bahan mengikut kumpulan:
(a) Elements of group 1, 2 and 13 – atoms of each element have 1, 2 and 3 valence electrons respectively are metals.
Unsur Kumpulan 1, 2 dan 13 – atom setiap unsur mempunyai 1, 2 dan 3 elektron valens adalah logam.
(b) The elements of group 3 to 12 – transition elements are metals.
Unsur Kumpulan 3 hingga 12 – unsur peralihan yang merupakan logam.
(c) The elements of Group 14, 15, 16, 17 and 18 – atoms of each element have 4, 5, 6, 7 and 8 valence electrons
respectively are non-metals.
Unsur Kumpulan 14, 15, 16, 17 dan 18 – atom setiap unsur mempunyai 4, 5, 6, 7 dan 8 elektron valens adalah bukan logam.
PERIOD / KALA
1 The horizontal row of elements in the Periodic Table, consists of the same number of shell occupied with
electrons in an atom called period.
Baris unsur secara mendatar dalam Jadual Berkala, mempunyai bilangan petala berisi elektron yang sama di
dalam atom disebut sebagai kala.
2 There are seven horizontal rows of elements known as Period 1, 2, ....., 7 [Refer to the Periodic Table]
Terdapat tujuh baris unsur secara mendatar disebut Kala 1, 2, ....., 7 [Rujuk Jadual Berkala]
#
(a) Period 1 has 2 elements / Kala 1 mengandungi 2 unsur Short periods, Period 3 will be studied in
(b) Period 2 and 3 have 8 elements / Kala 2 dan 3 mengandungi 8 unsur # detail with respect to physical and chemical
#
#
properties / Kala pendek, Kala 3 akan dipelajari
(c) Period 4 and 5 have 18 elements / Kala 4 dan 5 mengandungi 18 unsur dengan terperinci dari segi sifat fizik dan sifat kimia
(d) Period 6 has 32 elements / Kala 6 mengandungi 32 unsur Long periods / Kala panjang
(e) Period 7 has 23 elements / Kala 7 mengandungi 23 unsur
EXERCISE / LATIHAN
1 Complete the table below / Lengkapkan jadual berikut.
Element Proton number Electron arrangement Number of Group Number of shell Period
Unsur Nombor proton Susunan elektron valence electrons Kumpulan Bilangan petala Kala
Bilangan elektron
valens
H 1 1 1 1 1 1
He 2 2 2 18 1 1
Li 3 2.1 1 1 2 2
Be 4 2.2 2 2 2 2
B 5 2.3 3 13 2 2
C 6 2.4 4 14 2 2
N 7 2.5 5 15 2 2
O 8 2.6 6 16 2 2
F 9 2.7 7 17 2 2
Ne 10 2.8 8 18 2 2
Na 11 2.8.1 1 1 3 3
Mg 12 2.8.2 2 2 3 3
Al 13 2.8.3 3 13 3 3
52
Nilam Publication Sdn. Bhd.
03-Chem F4 (3P).indd 52 12/9/2011 5:57:52 PM