Page 52 - Nilam_Publication_module_Chemistry_Form.pdf
P. 52
MODULE • Chemistry Form 4
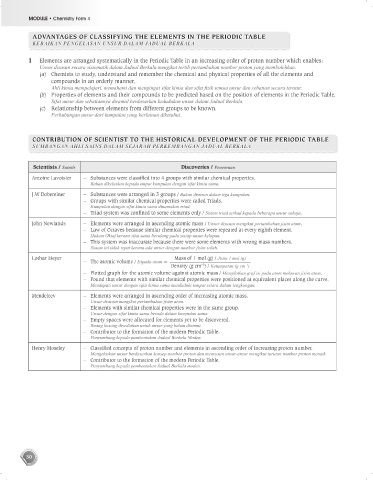
ADVANTAGES OF CLASSIFYING THE ELEMENTS IN THE PERIODIC TABLE
KEBAIKAN PENGELASAN UNSUR DALAM JADUAL BERKALA
1 Elements are arranged systematically in the Periodic Table in an increasing order of proton number which enables:
Unsur disusun secara sistematik dalam Jadual Berkala mengikut tertib pertambahan nombor proton yang membolehkan:
(a) Chemists to study, understand and remember the chemical and physical properties of all the elements and
compounds in an orderly manner,
Ahli kimia mempelajari, memahami dan mengingat sifat kimia dan sifat fizik semua unsur dan sebatian secara teratur.
(b) Properties of elements and their compounds to be predicted based on the position of elements in the Periodic Table,
Sifat unsur dan sebatiannya diramal berdasarkan kedudukan unsur dalam Jadual Berkala.
(c) Relationship between elements from different groups to be known.
Perhubungan unsur dari kumpulan yang berlainan diketahui.
CONTRIBUTION OF SCIENTIST TO THE HISTORICAL DEVELOPMENT OF THE PERIODIC TABLE
SUMBANGAN AHLI SAINS DALAM SEJARAH PERKEMBANGAN JADUAL BERKALA
Scientists / Saintis Discoveries / Penemuan
Antoine Lavoisier – Substances were classified into 4 groups with similar chemical properties.
Bahan dikelaskan kepada empat kumpulan dengan sifat kimia sama.
J.W Dobereiner – Substances were arranged in 3 groups / Bahan disusun dalam tiga kumpulan.
– Groups with similar chemical properties were called Triads.
Kumpulan dengan sifat kimia sama dinamakan triad.
– Triad system was confined to some elements only / Sistem triad terhad kepada beberapa unsur sahaja.
John Newlands – Elements were arranged in ascending atomic mass / Unsur disusun mengikut pertambahan jisim atom.
– Law of Octaves because similar chemical properties were repeated at every eighth element.
Hukum Oktaf kerana sifat sama berulang pada setiap unsur kelapan.
– This system was inaccurate because there were some elements with wrong mass numbers.
Sistem ini tidak tepat kerana ada unsur dengan nombor jisim salah.
Lothar Meyer Mass of 1 mol (g) / Jisim 1 mol (g)
– The atomic volume / Isipadu atom =
–3
–3
Density (g cm ) / Ketumpatan (g cm )
– Plotted graph for the atomic volume against atomic mass / Memplotkan graf isi padu atom melawan jisim atom.
– Found that elements with similar chemical properties were positioned at equivalent places along the curve.
Mendapati unsur dengan sifat kimia sama menduduki tempat setara dalam lengkungan.
Mendeleev – Elements were arranged in ascending order of increasing atomic mass.
Unsur disusun mengikut pertambahan jisim atom.
– Elements with similar chemical properties were in the same group.
Unsur dengan sifat kimia sama berada dalam kumpulan sama.
– Empty spaces were allocated for elements yet to be discovered.
Ruang kosong disediakan untuk unsur yang belum ditemui.
– Contributor to the formation of the modern Periodic Table.
Penyumbang kepada pambentukan Jadual Berkala Moden.
Henry Moseley – Classified concepts of proton number and elements in ascending order of increasing proton number.
Mengelaskan unsur berdasarkan konsep nombor proton dan menyusun unsur-unsur mengikut turutan nombor proton menaik.
– Contributor to the formation of the modern Periodic Table.
Penyumbang kepada pembentukan Jadual Berkala moden.
50
Nilam Publication Sdn. Bhd.
03-Chem F4 (3P).indd 50 12/9/2011 5:57:52 PM