Page 87 - Nilam_Publication_module_Chemistry_Form.pdf
P. 87
Chemistry Form 4 • MODULE
(d) Particle Y combines with particle Q to form a compound / Zarah Y bergabung dengan zarah Q untuk membentuk sebatian.
(i) State the type of compound formed / Nyatakan jenis sebatian yang terbentuk.
Ionic compound
(ii) Write chemical formula for the compound formed / Tuliskan formula kimia bagi sebatian yang terbentuk.
YQ
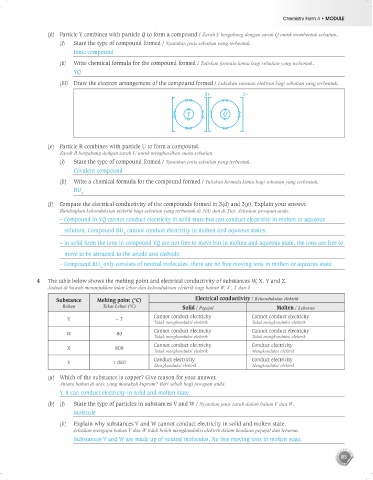
(iii) Draw the electron arrangement of the compound formed / Lukiskan susunan elektron bagi sebatian yang terbentuk.
2+ 2–
Y Q
(e) Particle R combines with particle U to form a compound.
Zarah R bergabung dengan zarah U untuk menghasilkan suatu sebatian.
(i) State the type of compound formed / Nyatakan jenis sebatian yang terbentuk.
Covalent compound
(ii) Write a chemical formula for the compound formed / Tuliskan formula kimia bagi sebatian yang terbentuk.
RU
4
(f) Compare the electrical conductivity of the compounds formed in 3(d) and 3(e). Explain your answer.
Bandingkan kekonduksian elektrik bagi sebatian yang terbentuk di 3(d) dan di 3(e). Jelaskan jawapan anda.
– Compound in YQ cannot conduct electricity in solid state but can conduct electricity in molten or aqueous
solution. Compound RU cannot conduct electricity in molten and aqueous states.
4
– In solid form the ions in compound YQ are not free to move but in molten and aqueous state, the ions are free to
move to be attracted to the anode and cathode.
– Compound RU only consists of neutral molecules, there are no free moving ions in molten or aqueous state.
4
4 The table below shows the melting point and electrical conductivity of substances W, X, Y and Z.
Jadual di bawah menunjukkan takat lebur dan kekonduksian elektrik bagi bahan W, X , Y dan Z.
Substance Melting point (°C) Electrical conductivity / Kekonduksian elektrik
Bahan Takat Lebur (°C) Solid / Pepejal Molten / Leburan
V – 7 Cannot conduct electricity Cannot conduct electricity
Tidak mengkonduksi elektrik Tidak mengkonduksi elektrik
Cannot conduct electricity Cannot conduct electricity
W 80
Tidak mengkonduksi elektrik Tidak mengkonduksi elektrik
Cannot conduct electricity Conduct electricity
X 808
Tidak mengkonduksi elektrik Mengkonduksi elektrik
Conduct electricity Conduct electricity
Y 1 080
Mengkonduksi elektrik Mengkonduksi elektrik
(a) Which of the substance is copper? Give reason for your answer.
Antara bahan di atas, yang manakah kuprum? Beri sebab bagi jawapan anda.
Y. It can conduct electricity in solid and molten state.
(b) (i) State the type of particles in substances V and W / Nyatakan jenis zarah dalam bahan V dan W.
Molecule
(ii) Explain why substances V and W cannot conduct electricity in solid and molten state.
Jelaskan mengapa bahan V dan W tidak boleh mengkonduksi elektrik dalam keadaan pepejal dan leburan.
Substances V and W are made up of neutral molecules. No free moving ions in molten state.
85
Nilam Publication Sdn. Bhd.
04-Chem F4 (3P).indd 85 12/9/2011 5:57:12 PM