Page 91 - Nilam_Publication_module_Chemistry_Form.pdf
P. 91
Chemistry Form 4 • MODULE
ELECTROLYSIS / ELEKTROLISIS
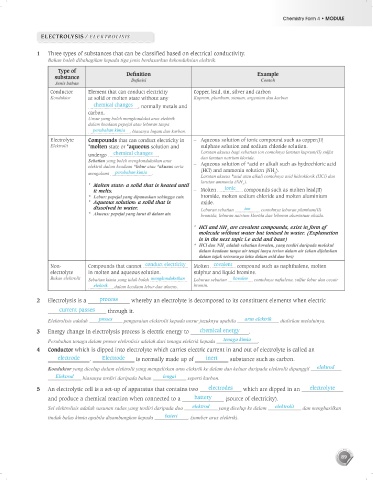
1 Three types of substances that can be classified based on electrical conductivity.
Bahan boleh dibahagikan kepada tiga jenis berdasarkan kekonduksian elektrik.
Type of
substance Def nition Example
Contoh
Definisi
Jenis bahan
Conductor Element that can conduct electricity Copper, lead, tin, silver and carbon
Konduktor at solid or molten state without any Kuprum, plumbum, stanum, argentum dan karbon
chemical changes , normally metals and
carbon.
Unsur yang boleh mengkonduksi arus elektrik
dalam keadaan pepejal atau leburan tanpa
perubahan kimia , biasanya logam dan karbon.
Electrolyte Compounds that can conduct electricity in – Aqueous solution of ionic compound such as copper(II)
Elektrolit *molten state or *aqueous solution and sulphate solution and sodium chloride solution.
undergo chemical changes . Larutan akueus bagi sebatian ion contohnya larutan kuprum(II) sulfat
Sebatian yang boleh mengkonduksikan arus – dan larutan natrium klorida.
Aqueous solution of *acid or alkali such as hydrochloric acid
elektrik dalam keadaan *lebur atau *akueus serta (HCl) and ammonia solution (NH ).
mengalami perubahan kimia . 3
Larutan akueus *asid atau alkali contohnya asid hidroklorik (HCl) dan
larutan ammonia (NH ).
* Molten state: a solid that is heated until ionic 3
it melts. – Molten compounds such as molten lead(II)
* Lebur: pepejal yang dipanaskan sehingga cair. bromide, molten sodium chloride and molten aluminium
* Aqueous solution: a solid that is oxide.
dissolved in water. Leburan sebatian ion contohnya leburan plumbum(II)
* Akueus: pepejal yang larut di dalam air. bromida, leburan natrium klorida dan leburan aluminium oksida.
* HCl and NH are covalent compounds, exist in form of
3
molecule without water but ionised in water. (Explanation
is in the next topic i.e acid and base)
* HCl dan NH adalah sebatian kovalen, yang terdiri daripada molekul
3
dalam keadaan tanpa air tetapi ianya terion dalam air (akan dijelaskan
dalam tajuk seterusnya iaitu dalam asid dan bes)
Non- Compounds that cannot conduct electricity Molten covalent compound such as naphthalene, molten
electrolyte in molten and aqueous solution. sulphur and liquid bromine.
Bukan elektrolit Sebatian kimia yang tidak boleh mengkonduksikan Leburan sebatian kovalen contohnya naftalena, sulfur lebur dan cecair
elektrik dalam keadaan lebur dan akueus. bromin.
2 Electrolysis is a process whereby an electrolyte is decomposed to its constituent elements when electric
current passes through it.
Elektrolisis adalah proses penguraian elektrolit kepada unsur juzuknya apabila arus elektrik dialirkan melaluinya.
3 Energy change in electrolysis process is electric energy to chemical energy .
Perubahan tenaga dalam proses elekrolisis adalah dari tenaga elektrik kepada tenaga kimia .
4 Conductor which is dipped into electrolyte which carries electric current in and out of electrolyte is called an
electrode . Electrode is normally made up of inert substance such as carbon.
Konduktor yang dicelup dalam elektrolit yang mengalirkan arus elektrik ke dalam dan keluar daripada elektrolit dipanggil elektrod .
Elektrod biasanya terdiri daripada bahan lengai seperti karbon.
5 An electrolytic cell is a set-up of apparatus that contains two electrodes which are dipped in an electrolyte
and produce a chemical reaction when connected to a battery (source of electricity).
Sel elektrolisis adalah susunan radas yang terdiri daripada dua elektrod yang dicelup ke dalam elektrolit dan menghasilkan
tindak balas kimia apabila disambungkan kepada bateri . (sumber arus elektrik).
89
Nilam Publication Sdn. Bhd.
05-Chem F4 (3P).indd 89 12/9/2011 5:56:27 PM