Page 22 - Jurnal Pendidikan Tingkatan Enam 2019 - Jilid 3
P. 22
Perubahan Paradigma Pemikiran Ke Arah Pendidikan Berkualiti
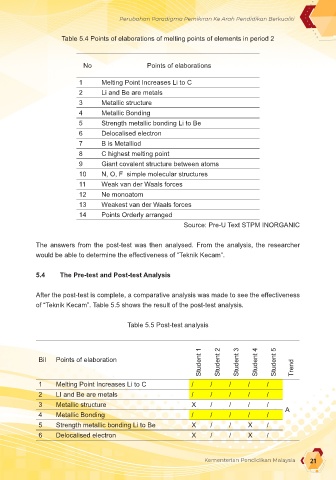
Table 5.4 Points of elaborations of melting points of elements in period 2
No Points of elaborations
1 Melting Point Increases Li to C
2 Li and Be are metals
3 Metallic structure
4 Metallic Bonding
5 Strength metallic bonding Li to Be
6 Delocalised electron
7 B is Metalliod
8 C highest melting point
9 Giant covalent structure between atoms
10 N, O, F simple molecular structures
11 Weak van der Waals forces
12 Ne monoatom
13 Weakest van der Waals forces
14 Points Orderly arranged
Source: Pre-U Text STPM INORGANIC
The answers from the post-test was then analysed. From the analysis, the researcher
would be able to determine the effectiveness of “Teknik Kecam”.
5.4 The Pre-test and Post-test Analysis
After the post-test is complete, a comparative analysis was made to see the effectiveness
of “Teknik Kecam”. Table 5.5 shows the result of the post-test analysis.
Table 5.5 Post-test analysis
Student 1 Student 2 Student 3 Student 4 Student 5 Trend
Bil Points of elaboration
1 Melting Point Increases Li to C / / / / /
2 LI and Be are metals / / / / /
3 Metallic structure X / / / / A
4 Metallic Bonding / / / / /
5 Strength metallic bonding Li to Be X / / X /
6 Delocalised electron X / / X /
Kementerian Pendidikan Malaysia 21