Page 135 - Basic Principles of Textile Coloration
P. 135
124 PROTEIN FIBRES
solution of the reducing agent sodium bisulphite removes residual traces of
chlorine (Scheme 7.4). Both chlorination methods, however, require careful
control to ensure uniformity of the treatment and to avoid imparting an overly
harsh handle to the wool. The reaction of chlorine on the cuticle is very rapid. It is
for this reason that the chlorine must be generated or added slowly.
The chlorination process increases the substantivity of acid dyes for the wool
and level dyeing becomes more difficult. It will be impossible if the wool has been
unevenly chlorinated, since chlorinated wool has a much higher rate of dye
absorption. Unfortunately, chlorinated wool also gives much higher rates of dye
desorption on washing. Acid dyes with good migration, which might be useful for
dyeing unevenly chlorinated wool because of desorption from heavily dyed fibres
and re-absorption by paler ones, also have rather poor washing fastness and are
almost useless on chlorinated wool that will be washed regularly.
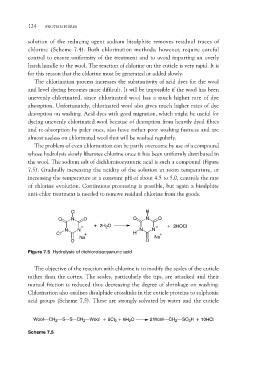
The problem of even chlorination can be partly overcome by use of a compound
whose hydrolysis slowly liberates chlorine once it has been uniformly distributed in
the wool. The sodium salt of dichloroisocyanuric acid is such a compound (Figure
7.5). Gradually increasing the acidity of the solution at room temperature, or
increasing the temperature at a constant pH of about 4.5 to 5.0, controls the rate
of chlorine evolution. Continuous processing is possible, but again a bisulphite
anti-chlor treatment is needed to remove residual chlorine from the goods.
Cl + 2H2O H + 2HOCl
ONO ONO
NN NN
Cl H
Na Na
O O
Figure 7.5 Hydrolysis of dichloroisocyanuric acid
The objective of the reaction with chlorine is to modify the scales of the cuticle
rather than the cortex. The scales, particularly the tips, are attacked and their
mutual friction is reduced thus decreasing the degree of shrinkage on washing.
Chlorination also oxidises disulphide crosslinks in the cuticle proteins to sulphonic
acid groups (Scheme 7.5). These are strongly solvated by water and the cuticle
Wool CH2 S S CH2 Wool + 5Cl2 + 6H2O 2 Wool CH2 SO3H + 10HCl
Scheme 7.5