Page 14 - Basic Principles of Textile Coloration
P. 14
HISTORICAL BACKGROUND 3
Insoluble vat reduction Soluble Leuco oxidation Insoluble
dye pigment in leuco compound vat dye
aqueous compound absorbed in pigment held
suspension in solution the fibres in the fibres
Scheme 1.1
1.1.2 The development of synthetic dyes and fibres
In 1856, William H Perkin reacted aniline with acidic potassium dichromate
solution in an attempt to prepare the anti-malarial drug quinine. From the dark,
tarry reaction mixture, he isolated a purple, water-soluble compound that dyed
both wool and silk directly when immersed in its solution. No mordant was
required. Perkin established a factory for the large-scale production of aniline and
for the manufacture of this dye, later called Mauveine. He not only discovered the
first major synthetic dye, but founded the modern chemical industry.
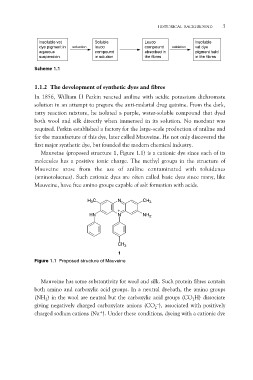
Mauveine (proposed structure 1, Figure 1.1) is a cationic dye since each of its
molecules has a positive ionic charge. The methyl groups in the structure of
Mauveine arose from the use of aniline contaminated with toluidenes
(aminotoluenes). Such cationic dyes are often called basic dyes since many, like
Mauveine, have free amino groups capable of salt formation with acids.
H3C N CH3
HN N NH2
CH3
1
Figure 1.1 Proposed structure of Mauveine
Mauveine has some substantivity for wool and silk. Such protein fibres contain
both amino and carboxylic acid groups. In a neutral dyebath, the amino groups
(NH2) in the wool are neutral but the carboxylic acid groups (CO2H) dissociate
giving negatively charged carboxylate anions (CO2–), associated with positively
charged sodium cations (Na+). Under these conditions, dyeing with a cationic dye