Page 16 - Basic Principles of Textile Coloration
P. 16
HISTORICAL BACKGROUND 5
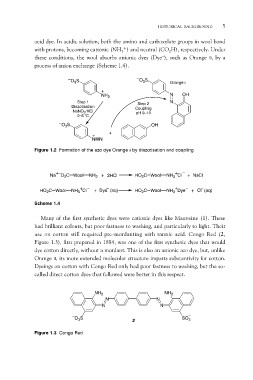
acid dye. In acidic solution, both the amino and carboxylate groups in wool bond
with protons, becoming cationic (NH3+) and neutral (CO2H), respectively. Under
these conditions, the wool absorbs anionic dyes (Dye–), such as Orange II, by a
process of anion exchange (Scheme 1.4).
O3S O3S Orange II
NH3 N OH
N
Step 1 Step 2
Diazotisation Coupling
NaNO2/HCl pH 9–10
0–5 °C OH
O3S
+
NN
Figure 1.2 Formation of the azo dye Orange II by diazotisation and coupling
Na+_O2C Wool NH2 + 2HCl HO2C Wool NH3+Cl _ + NaCl
HO2C Wool NH3+Cl _ _ HO2C Wool NH3+Dye_ + _
+ Dye (aq) Cl (aq)
Scheme 1.4
Many of the first synthetic dyes were cationic dyes like Mauveine (1). These
had brilliant colours, but poor fastness to washing, and particularly to light. Their
use on cotton still required pre-mordanting with tannic acid. Congo Red (2,
Figure 1.3), first prepared in 1884, was one of the first synthetic dyes that would
dye cotton directly, without a mordant. This is also an anionic azo dye, but, unlike
Orange II, its more extended molecular structure imparts substantivity for cotton.
Dyeings on cotton with Congo Red only had poor fastness to washing, but the so-
called direct cotton dyes that followed were better in this respect.
NH2 NH2
N N
N N
O3S 2 SO3
Figure 1.3 Congo Red