Page 19 - Basic Principles of Textile Coloration
P. 19
8 AN INTRODUCTION TO TEXTILES, DYES AND DYEING
acetic anhydride to produce cellulose acetates (Scheme 1.6). In 1921, a cellulose
acetate fibre was produced with about 80% of the cellulose hydroxyl groups
acetylated. This cellulose acetate gave silky, lustrous filaments on extrusion of its
acetone solution followed by immediate evaporation of the solvent. These
filaments were quite different from cotton or viscose. In particular, they were
relatively hydrophobic (water-repelling), whereas cotton and viscose are
hydrophilic (water-attracting). Initially, cellulose acetate proved difficult to dye
satisfactorily with existing ionic dyes. Effective dyeing occurred, however, using a
fine aqueous dispersion of non-ionic, relatively insoluble, hydrophobic dyes. This
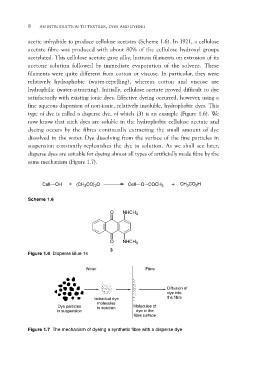
type of dye is called a disperse dye, of which (3) is an example (Figure 1.6). We
now know that such dyes are soluble in the hydrophobic cellulose acetate and
dyeing occurs by the fibres continually extracting the small amount of dye
dissolved in the water. Dye dissolving from the surface of the fine particles in
suspension constantly replenishes the dye in solution. As we shall see later,
disperse dyes are suitable for dyeing almost all types of artificially made fibre by the
same mechanism (Figure 1.7).
Cell OH + (CH3CO)2O Cell O COCH3 + CH3CO2H
Scheme 1.6
O NHCH3
Figure 1.6 Disperse Blue 14 O NHCH3
3
Water
Fibre
Individual dye Diffusion of
molecules dye into
in solution the fibre
Dye particles Molecules of
in suspension dye in the
fibre surface
Figure 1.7 The mechanism of dyeing a synthetic fibre with a disperse dye