Page 216 - Basic Principles of Textile Coloration
P. 216
DYEING EQUILIBRIA 205
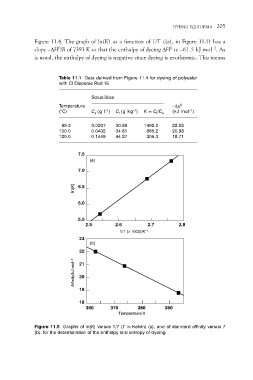
Figure 11.4. The graph of ln(K) as a function of 1/T ((a), in Figure 11.5) has a
slope –DH0/R of 7393 K so that the enthalpy of dyeing DH0 is –61.5 kJ mol–1. As
is usual, the enthalpy of dyeing is negative since dyeing is exothermic. This means
Table 11.1 Data derived from Figure 11.4 for dyeing of polyester
with CI Disperse Red 15
Temperature Solubilities K = Cf/Cs –Dm0
(°C) Cs ( g l–1) Cf (g kg–1) (kJ mol–1)
89.3 0.0207 30.89 1492.0 22.02
100.0 0.0402 34.81 865.2 20.98
120.0 0.1449 44.27 305.3 18.71
7.5
(a)
7.0
In(K) 6.5
6.0
5.5 2.6 2.7 2.8
2.5 1/T ( 1000)/K–1
23
(b)
22
Affinity/kJ mol–1 21
20
19
18 370 380 390
360 Temperature/K
Figure 11.5 Graphs of ln(K) versus 1/T (T in Kelvin) (a), and of standard affinity versus T
(b), for the determination of the enthalpy and entropy of dyeing