Page 222 - Basic Principles of Textile Coloration
P. 222
DYEING KINETICS 211
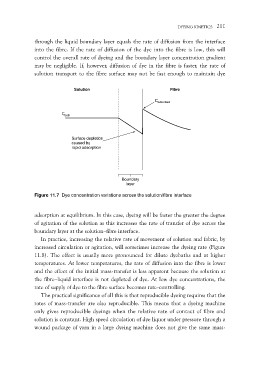
through the liquid boundary layer equals the rate of diffusion from the interface
into the fibre. If the rate of diffusion of the dye into the fibre is low, this will
control the overall rate of dyeing and the boundary layer concentration gradient
may be negligible. If, however, diffusion of dye in the fibre is faster, the rate of
solution transport to the fibre surface may not be fast enough to maintain dye
Solution Fibre
Cbath Cadsorbed
Surface depletion
caused by
rapid adsorption
Boundary
layer
Figure 11.7 Dye concentration variations across the solution/fibre interface
adsorption at equilibrium. In this case, dyeing will be faster the greater the degree
of agitation of the solution as this increases the rate of transfer of dye across the
boundary layer at the solution–fibre interface.
In practice, increasing the relative rate of movement of solution and fabric, by
increased circulation or agitation, will sometimes increase the dyeing rate (Figure
11.8). The effect is usually more pronounced for dilute dyebaths and at higher
temperatures. At lower temperatures, the rate of diffusion into the fibre is lower
and the effect of the initial mass-transfer is less apparent because the solution at
the fibre–liquid interface is not depleted of dye. At low dye concentrations, the
rate of supply of dye to the fibre surface becomes rate-controlling.
The practical significance of all this is that reproducible dyeing requires that the
rates of mass-transfer are also reproducible. This means that a dyeing machine
only gives reproducible dyeings when the relative rate of contact of fibre and
solution is constant. High speed circulation of dye liquor under pressure through a
wound package of yarn in a large dyeing machine does not give the same mass-